Moderna said it has collated sufficient data for the first interim analysis of the Phase 3 study of its COVID-19 vaccine candidate and is on track to submit the findings as early as this month. Shares rose 2.2% in Wednesday’s extended market trading after closing 8.4% higher on the day.
Moderna (MRNA) said that it has completed case accrual for the first interim analysis of the Phase 3 trial of its mRNA-1273 candidate and that the data on these cases is being prepared for submission to the independent Data Safety Monitoring Board (DSMB) for analysis and recommendation. At the end of October, Moderna said that it expected first interim data analysis to be released sometime this month after having already received $1.1 billion in cash payments for the supply of its COVID-19 vaccine candidate.
The company’s mRNA-1273 candidate is an mRNA vaccine against COVID-19 encoding for a prefusion stabilized form of the Spike (S) protein and is being co-developed with researchers from the National Institute of Allergy and Infectious Disease’s (NIAID) Vaccine Research Center.
Moderna disclosed that it has seen a significant increase in the number of COVID-19 infected participants across trial sites over the last week as the US is seeing a resurgence in cases. As a result, the company expects the first interim analysis to include “substantially” more than the 53 infected participants, which had been the targeted trigger point for the analysis.
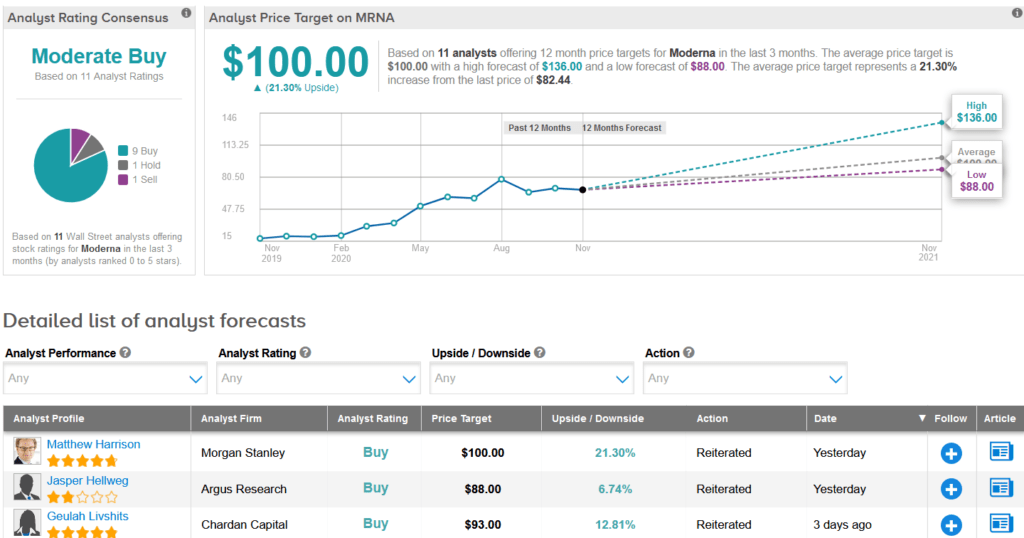
Shares in Moderna have surged more than 18% over the past five days and are up a stellar 321% so far this year. Wall Street analysts still have a Moderate Buy consensus on the stock’s outlook. Looking ahead, the average price target stands at $100, indicating another 21% upside potential lies ahead.
Following Moderna’s update, Argus analyst Jasper Hellweg raised the stock’s price target to $88 from $80 and maintained a Buy rating, citing the company’s continued vaccine progress as it has recently completed the enrollment of 30,000 participants for its Phase 3 study of mRNA-1273.
Hellweg noted that following the accrual and assessment of two months of safety and follow-up data, the company’s next step will be to determine whether to submit an application to the US Food and Drug Administration (FDA) to request Emergency Use Authorization (EUA). The analyst is also encouraged that Moderna has already inked agreements with multiple governments globally to provide initial doses of mRNA-1273 upon its regulatory approval. (See MRNA stock analysis on TipRanks).
Related News:
Pfizer, BioNTech Announce COVID-19 Vaccine is 90% Effective
Ocular Therapeutix Rises 7% On Stellar Results Backed By Dextenza
Canada’s Canopy Growth Pops 12% On Blowout Quarter, Cost Cuts









