Shares of Pfizer (PFE) and BioNTech (BNTX) jumped over 12% and 27%, respectively, in pre-market trading on November 9 after the companies published data suggesting their mRNA-based COVID-19 vaccine candidate is more than 90% effective in preventing COVID-19 in subjects who had no evidence of a previous infection.
“This is a historical moment… This was a devastating situation, a pandemic, and we have embarked on a path and a goal that nobody ever has achieved — to come up with a vaccine within a year,” Kathrin Jansen, a senior vice president and the head of vaccine research and development at Pfizer, said.
The first interim efficacy analysis was conducted by an independent Data Monitoring Committee from the Phase 3 clinical study, with the analysis evaluating 94 confirmed cases of COVID-19 among the trial’s 43,538 subjects. Both companies stated that the case split between vaccinated participants and those who were dosed with a placebo implied a vaccine efficacy rate of over 90% at seven days after the second dose. Management also said that no serious adverse events have been witnessed.
A 90% level of protection goes head-to-head with highly effective childhood vaccines for conditions like measles.
This encouraging update comes as researchers and healthcare companies race to develop a vaccine to combat the virus that has already claimed over 1.2 million lives.
Scientists had been hoping for a vaccine that was 75% effective, with Dr. Anthony Fauci, the director of the National Institute of Allergy and Infectious Diseases, previously noting a vaccine that was 50%-60% effective would be sufficient.
Pfizer expects to file for an Emergency Use Authorization (EUA) with the FDA later this month, after it has gathered the recommended two months of safety data. It also plans to manufacture enough doses of the vaccine for 15-20 million people, based on commentary from management.
“With today’s news, we are a significant step closer to providing people around the world with a much-needed breakthrough to help bring an end to this global health crisis. We look forward to sharing additional efficacy and safety data generated from thousands of participants in the coming weeks,” Pfizer Chairman and CEO Dr. Albert Bourla commented.
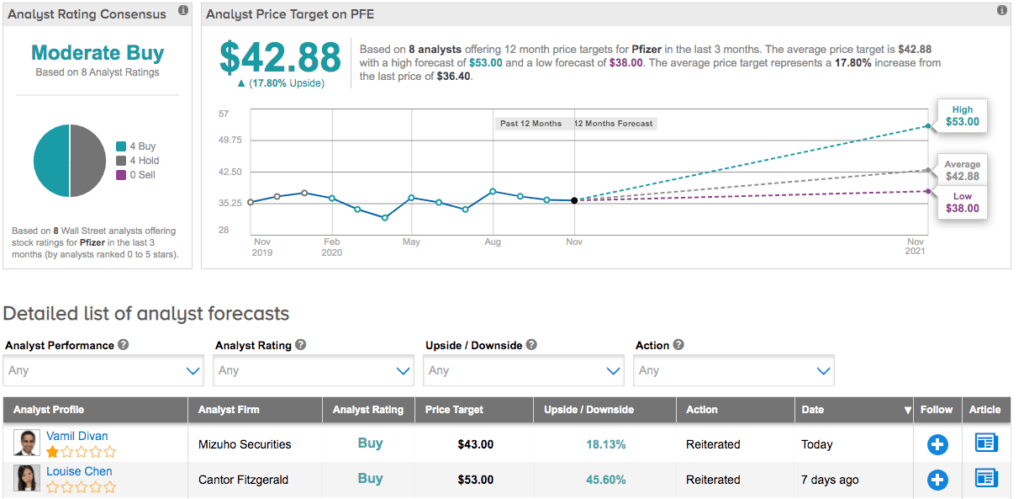
Shares of Pfizer are down 7% year-to-date, but Wall Street is cautiously optimistic. Its Moderate Buy analyst consensus breaks down into 4 Buys and 4 Holds. At $42.88, the average price target puts the upside potential at 18%. (See Pfizer stock analysis on TipRanks)
In response to the news, Mizuho Securities analyst Vamil Divan reiterated a Buy rating and $43 price target, arguing the fact that Pfizer reported a larger number of events than expected should be seen as a positive, with potential vaccine sales surpassing $8.5 billion between 2020-2021. He added, “The speed with which Pfizer has moved to develop this vaccine candidate is also encouraging to us, and suggests Pfizer may be able to meet its stated objectives of being a faster moving, more nimble biopharmaceutical company.”
Related News:
Ocular Therapeutix Rises 7% On Stellar Results Backed By Dextenza
Canada’s Canopy Growth Pops 12% On Blowout Quarter, Cost Cuts
AstraZeneca’s Heart Drug Gets FDA Nod For Stroke Reduction Use









