Pfizer and German partner BioNTech will start distribution of the first doses of its BNT162b2 in Switzerland after the country’s regulatory agency granted authorization of the COVID-19 vaccine for individuals aged 16 and older.
Swissmedic, the Swiss Agency for Therapeutic Products, said that the approval of the Pfizer (PFE) vaccine marks the world’s first authorization in the ordinary procedure. According to the data assessed by the Swiss agency, the level of protection demonstrated seven days after the second injection of the vaccine is over 90%. The data available to date showed a comparable high level of efficacy in all investigated age groups, thus meeting the safety requirements, the agency added.
Overall, Swissmedic concluded that the Pfizer-BioNTech’s COVID-19 vaccine is safe and that its benefit outweighs the risks. Switzerland has a supply contract with Pfizer and BioNTech for the delivery of 3 million doses of the vaccine.
“The safety of patients is an essential prerequisite, especially where the authorisation of vaccines is concerned”, commented Swissmedic Director Raimund Bruhin. “Thanks to the rolling procedure and our flexibly organised teams, we nevertheless managed to reach a decision quickly – while also fully satisfying the three most important requirements of safety, efficacy and quality.”
The authorization application for BNT162b2, an mRNA-based vaccine, was submitted to the Swiss watchdog in mid-October, and was reviewed on an ongoing rolling basis. Earlier this month, the Pfizer-BioNTech COVID-19 vaccine was authorized for emergency use by the US Food and Drug Administration to prevent COVID-19 in individuals aged 16 years and older.
In a separate statement, Pfizer rebuffed recent public comments that alleged that the US drugmaker is grappling with issues in the production and distribution of its COVID-19 vaccine.
“Pfizer is not having any production issues with our COVID-19 vaccine, and no shipments containing the vaccine are on hold or delayed,” the company stated on Dec. 17. “This week, we successfully shipped all 2.9 million doses that we were asked to ship by the U.S. Government to the locations specified by them. We have millions more doses sitting in our warehouse but, as of now, we have not received any shipment instructions for additional doses.”
Additionally, Pfizer confirmed that it remained on track to deliver up to 50 million vaccine doses globally this year and up to 1.3 billion next year.
Shares of Pfizer have declined 8.4% over the past month but are up 1.5% on a year-to-date basis. (See Pfizer stock analysis on TipRanks)
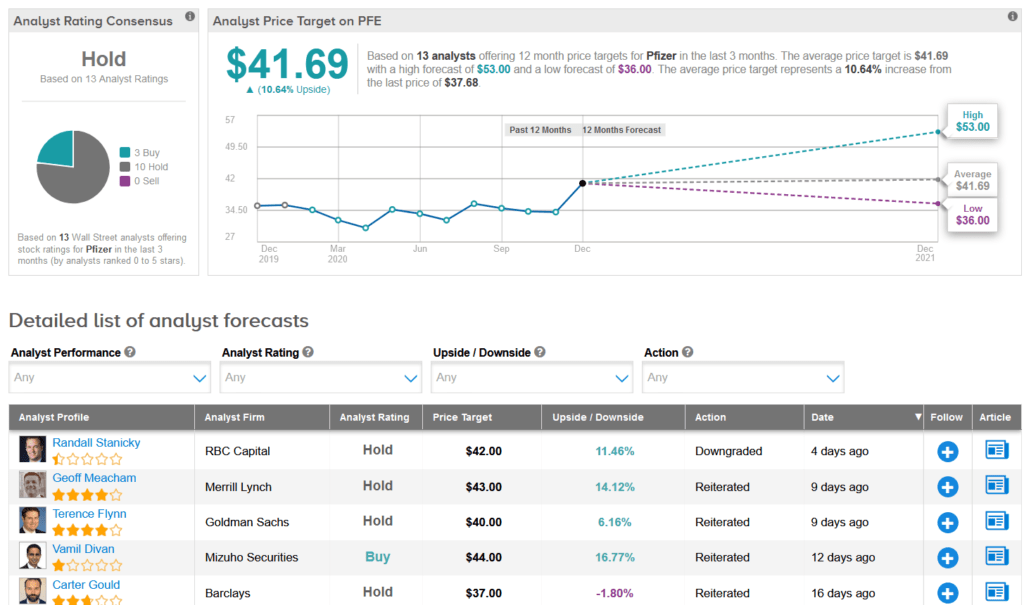
Last week, RBC Capital analyst Randall Stanicky downgraded PFE to Hold from Buy and lowered the price target to $42 from $43, as he argued that the share price already reflected the recent COVID-19 vaccine approval milestone, which would make additional upside harder to justify.
“We see it as likely rangebound near-term,” Stanicky wrote in a note to investors, adding that fierce competition from other drugmakers in the COVID-19 vaccine space “could catch up” in 2021.
Overall, the rest of the Street is sidelined on the stock with a Hold analyst consensus. That’s based on 10 Holds vs. 3 Buys. Looking ahead, the average analyst price target stands at $41.69, putting the upside potential at about 11% over the next 12 months.
Related News:
Moderna’s Covid-19 Vaccine Wins FDA Emergency Use Approval
Mesoblast Fails to Meet Primary Endpoint in COVID-19 Trial; Street Sees 29% Upside
FedEx Shares Fall 4% Despite 2Q Earnings Beat; Analysts Stay Bullish









