Shares of Hepion Pharmaceuticals exploded 47% in Wednesday’s pre-market trading after the biopharma company released “positive” top line trial results for its oral inhibitor used in the treatment of an advanced form of non-alcoholic fatty liver disease.
Specifically, Hepion (HEPA) announced that the results from the low dose cohort in the company’s Phase 2a clinical trial showed that CRV431, an oral, once daily cyclophilin inhibitor, was generally safe and well tolerated and demonstrated clinically significant reductions in important biomarkers.
The pharmacokinetics further indicated that blood concentrations of CRV431 were similar to those observed in the earlier Phase 1 studies in healthy volunteers. Following the findings, the Phase 2a study is now being continued with the higher dose of 225 mg CRV431. The dosing of the patients suffering from non-alcoholic steatohepatitis (NASH) is expected to be completed in the first quarter of 2021.
Hepion is a clinical stage biopharmaceutical company focused on artificial intelligence-driven therapeutic drug development for the treatment of NASH and other liver diseases. CRV431 has been shown to reduce liver fibrosis and hepatocellular carcinoma tumor burden in experimental models of NASH, the company said. In preclinical studies, the company’s lead drug candidate has demonstrated antiviral responses towards HBV, HCV, and HDV.
“We are pleased to see this positive movement in liver chemistry tests,” commented Robert Foster, Hepion’s CEO. “Although the primary focus of this Phase 2a study was to examine the safety and tolerability of CRV431 in NASH patients, we were hoping to see early signs of potential efficacy and are pleased with the results thus far. We will continue to monitor liver safety lab tests in our higher dose group and will also look at a panel of serum fibrosis biomarkers.”
Foster added that he expected to initiate a Phase 2b trial midway through 2021.
The trial results were based on a placebo-controlled study of CRV431 in NASH patients with moderate-to-severe fibrosis. In this study, which is being conducted at 10 US sites, a low dose of 75 mg CRV431 was administered orally, once-daily for 28 days. A second dosing cohort of 225 mg CRV431 is ongoing. Final results from both dosing cohorts are expected after the high dose group has completed active dosing, followed by a 14-day observation period.
The primary objectives of the trial are to assess safety and tolerability of CRV431, as well as to delineate pharmacokinetics. The secondary outcome measure of the Phase 2a trial is to evaluate decreases in antifibrotic markers from baseline to the end of study.
Hepion shares have spiked 24% over the past month but are still down 61% on a year-to-date basis. (See HEPA stock analysis on TipRanks)
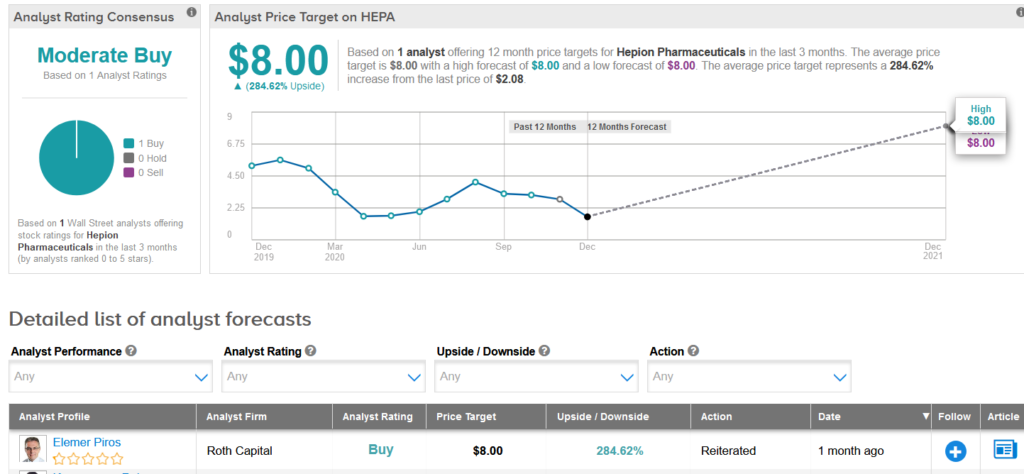
The stock’s coverage has recently been reviewed by Roth Capital analyst Elemer Piros, who reiterated a Buy rating.
However, Piros slashed his price target to $8 (285% upside potential) from $14, after Hepion’s recent closure of a follow-on offering of 23 million shares priced at $1.50 a share, which caused a significantly higher dilution than he had previously expected.
Related News:
Pfizer, BioNTech to Deliver Additional 100M Vaccine Doses to EU
Quanterix Wins FDA Approval For Covid-19 Antibody Test; Shares Rise 6%
Arcturus Gets Approval For Covid-19 Vaccine Phase 2 Study; Shares Plunge 38%