COVID-19 might not be going anywhere anytime soon. Last week, Florida, Texas, California and Arizona all reported that the daily rate of new infections had spiked. Heightened fears of a second wave and the possible reversal of reopening measures spooked the market, with the three major U.S. stock indexes landing in the red on June 26.
The resiliency of the virus has understandably alarmed investors, as the pandemic has devasted certain areas of the market. That being said, others have boomed during these tumultuous times. Looking specifically at the biotech sector, massive amounts of capital have been pumped into a handful of names racing to develop solutions to combat the virus. In the last three months, the NASDAQ Biotechnology Index (NBI) has climbed 29% higher, leaving the S&P 500’s 18% gain in the dust.
As some Wall Street analysts believe that a few of these coronavirus stocks still have plenty of room to grow, we took advantage of TipRanks’ database to get the lowdown on three of them. The platform revealed that each has earned a “Strong Buy” consensus rating from the analyst community and boasts substantial upside potential.
Co-Diagnostics Inc. (CODX)
Hoping to bring high-quality molecular diagnostics for infectious diseases to market, Co-Diagnostics uses unique technology that allows for more affordable testing, research and design solutions. Based on the potential of its Logix Smart COVID-19 test, this name has landed under Wall Street’s microscope.
Writing for H.C. Wainwright, 5-star analyst Yi Chen tells clients that he has been impressed by the company, to say the least. On May 26, CODX announced that its product had been able to detect SARS-CoV-2 in formalin-fixed paraffin-embedded (FFPE) samples from surgical resection of tongue squamous cell carcinoma in a patient who developed COVID-19 after surgery. The analyst noted, “RNA of SARS-CoV-2 strain was detected in the tumor and the normal submandibular gland samples using the Logix Smart COVID-19 real-time PCR test, but no viral RNA was found in metastatic and reactive lymph nodes.”
What is the implication of these results? Chen stated, “The results demonstrated that SARS-CoV-2 RNA can be detected in routine histopathological samples even before COVID-19 disease development…The published results bode well for the test’s market prospects in the coming months, in our view, as employers around the world are planning to test asymptomatic employees to allow them to return to the workplace.”
The test’s impressive capabilities speak to the strength of CODX’s CoPrimer technology, “which effectively eliminates the propagation of primer-dimers normally found in PCR and increases the test’s sensitivity and specificity”, in Chen’s opinion.
On top of this, the biotech is developing additional tests to address the other obstacles in the diagnosis of a COVID-19 infection. These include a multiplex panel to distinguish between the COVID-19 virus and other upper respiratory pathogens, a test for the D614G mutation in SARS-CoV-2, which has been the most common strain in the U.S. as well as a test using CoPrimers to identify both the virus and the antibody associated with a past infection in one test.
“In our view, these new tests could differentiate the company’s offerings from other PCR tests and further drive market adoption of CoPrimer-based COVID-19 testing,” Chen commented.
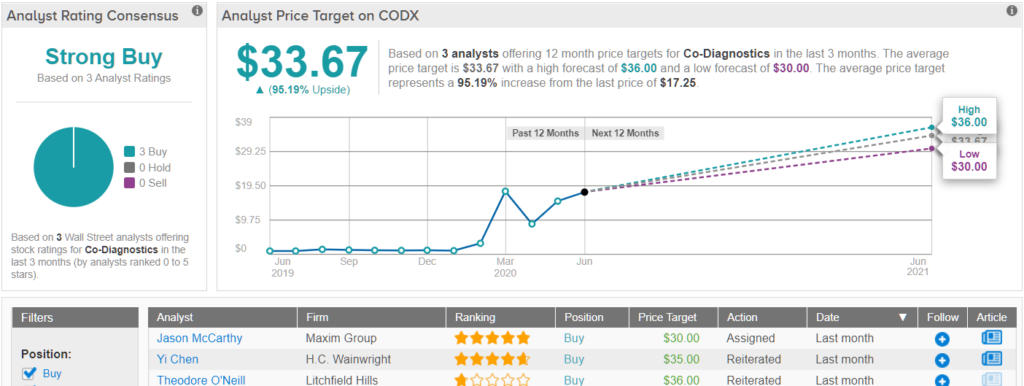
With CODX also boasting more than $100 million worth of tests as of mid-May that should be shipped to customers in the coming months, the deal is sealed for Chen. In addition to reiterating a Buy recommendation, he kept a $35 price target on the stock, implying 103% upside potential. (To watch Chen’s track record, click here)
Do other analysts agree with Chen? They do. Only Buy ratings, 3, in fact, have been issued in the last three months, so the consensus rating is a Strong Buy. At $33.67, the average price target brings the potential twelve-month gain to 95%. (See CODX stock analysis on TipRanks)
Kiniksa Pharmaceuticals (KNSA)
Primarily focused on developing therapeutic medicines designed to modulate immunological signaling pathways, Kiniksa wants to address the unmet medical needs of patients. Its potential COVID-19 treatment, mavrilimumab (mavri), has already produced promising results, with several members of the Street now singing its praises.
Based on new data released from an open-label, single-active arm, single center study in Italy in non-mechanically ventilated patients with severe COVID-19 pneumonia and hyperinflammation, patients given KNSA’s candidate saw a significant clinical improvement.
Highlighting the details of the study, JMP analyst Liisa Bayko pointed out that each patient was given medical treatment hydroxychloroquine, azithromycin and lopinavir/ritonavir as well as respiratory support with supplemental oxygen and/or non-invasive ventilation with continuous positive airway pressure open admission. It should also be noted that the study featured 13 patients treated with a single IV dose of mavrilimumab, compared to 26 contemporaneous patients that received local standard of care.
“Twice as many patients on mavri showed clinical improvement vs. control subjects and that clinical improvement happened earlier on mavri (median 8 days vs. not estimable). There were no deaths on mavri vs. 27% for control and only 8% of mavri patients required ventilation vs. 5% for the control group. Mavri patients also were discharged earlier as well,” Bayko commented.
As for what it all means, the five-star analyst said, “The data looks positive to us with standard caveats of small numbers and design limitations… The data continues to support our belief that mavrilimumab’s mechanism of action is well suited to treat COVID-19 patients.”
Going forward, KNSA is discussing a registrational program for mavri with the FDA, and investigator-initiated, placebo-controlled studies are being planned in both the U.S. and Italy.
Everything that KNSA has going for it prompted Bayko to stay with the bulls. Along with a Market Outperform rating, she maintained her $40 price target. This target implies shares could climb 55% higher in the next year. (To watch Bayko’s track record, click here)
Like Bayko, other analysts also take a bullish approach. KNSA’s Strong Buy consensus rating breaks down into 5 Buys and zero Holds or Sells. Given the $31 average price target, the upside potential lands at 32%. (See Kiniksa stock analysis on TipRanks)

Moderna, Inc. (MRNA)
Last but not least, we come across Moderna, which has grabbed headlines left and right as a result of its experimental COVID-19 vaccine, mRNA-1273.
Representing Piper Sandler, 5-star analyst Edward Tenthoff has high hopes based on early data. After MRNA published a preprint of mRNA-1273 preclinical data while simultaneously under peer-review for publishing in a leading medical journal, the analyst tells investors that the data suggests “mRNA-1273 supports clinical activity in humans.”
“mRNA-1273 formulated in lipid nanoparticles elicited a balance both humoral (antibody) and cellular (CD8 T cell) immunity. Mice immunized with 0.0025-20mcg of mRNA-1273 demonstrated strong dose-dependent correlation between binding and neutralizing antibody responses. Importantly, mice receiving two 1.0mcg doses of mRNA-1273 were completely protected from viral replication in lungs and nasal cavities after re-challenge at 5- and 13-weeks following boost,” Tenthoff explained.
Looking more closely at the candidate, it targets a pre-fusion Spike protein stabilized by two proline substitutions. This is important as it could allow for a more generalizable approach to be used to protect against mutations.
It should also be noted that the Phase 2 study of mRNA-1273 has already enrolled 300 healthy adults aged 18-55 years, and 50 out of 300 subjects over the age of 55. According to Tenthoff, the data from this study should “further validate safety and efficacy of the 100µg dose in a broader population, which was selected for the Phase 3 trial.” This trial is set to kick off in July.
Tenthoff added, “Lonza will support manufacturing of 500 million-1 billion doses per year. mRNA-1273 holds Fast Track Designation.”
Based on all of the above, it’s no wonder Tenthoff reiterated his Overweight rating. With a $100 price target, shares could gain 61% in the next twelve months. (To watch Tenthoff’s track record, click here)
Looking at the consensus breakdown, other analysts are on the same page. With 11 Buys and 2 Holds, the word on the Street is that MRNA is a Strong Buy. The $87.64 average price target puts the upside potential at 42%. (See Moderna stock-price forecast on TipRanks)
To find good ideas for coronavirus stocks trading at attractive valuations, visit TipRanks’ Best Stocks to Buy, a newly launched tool that unites all of TipRanks’ equity insights.