Shares of specialty vaccine maker Valneva (NASDAQ:VALN) are tanking today after the U.S. Food and Drug Administration revised the PDUFA (Prescription Drug User Fee Act) action date for the company’s chikungunya vaccine candidate VLA1553 to the end of November from the previous end of August.
Pick the best stocks and maximize your portfolio:
- Discover top-rated stocks from highly ranked analysts with Analyst Top Stocks!
- Easily identify outperforming stocks and invest smarter with Top Smart Score Stocks
The healthcare regulator has extended the action date to gain sufficient time to align and agree on the Phase 4 program for the candidate. The vaccine candidate is under the accelerated approval mechanism and importantly, the FDA has not requested any additional data for the approval.
If approved, VLA1553 could potentially be the first chikungunya vaccine on the market and Valneva is aiming to cooperate with FDA in its review of the biologics license application. Moreover, despite this extension in the timeline, the company has reiterated its guidance for a BLA approval and initial launch this year itself.
Further, Valneva is staying on course for regulatory submission in Canada and a planned submission in Europe.
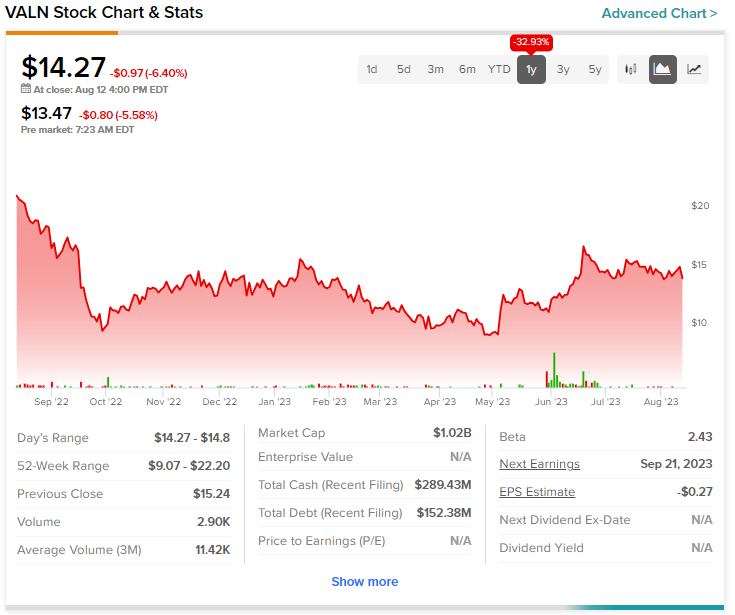
Today’s price erosion comes on top of a 33% decline in Valneva shares over the past year. Meanwhile, the Street has a Moderate Buy consensus rating on the stock alongside a $22 consensus price target.
Read full Disclosure



















