Novavax’s (NASDAQ: NVAX) COVID-19 vaccine (NVX-CoV2373) for individuals aged 18 years and above received the most awaited positive recommendation by the U.S. Food and Drug Administration (FDA) Vaccines and Related Biological Products Advisory Committee (VRBPAC).
Following the update, shares of the biotech firm rose more than 7% in pre-market trading at last glance.
The FDA’s advisory panel overwhelmingly recommended granting Emergency Use Authorization (EUA) for NVX-CoV2373, Novavax’s recombinant nanoparticle protein-based COVID-19 vaccine with Matrix-M adjuvant, as the primary vaccine for adults. The decision was favored by all members of the panel except one, who abstained.
This will be the first protein-based COVID vaccine, if authorized, and will be an alternative to the mRNA technology-based vaccines of BioNTech/Pfizer and Moderna (MRNA).
Supporting Data
The panel’s decision was supported by data from the pivotal Phase 3 clinical trial, PREVENT-19. The clinical trial included around 30,000 individuals aged 18 years and older in the U.S. and Mexico. Results demonstrated 90.4% efficacy of the vaccine, with a well-tolerated safety profile.
Also, regulators’ concern related to myocarditis (inflammation of the heart muscle) caused by the vaccine was subsidized as the data revealed a balanced rate of myocarditis between the vaccine and placebo arms.
Official Comments
Novavax CEO Stanley C. Erck commented, “The advisory committee’s positive recommendation acknowledges the strength of our data and the importance of a protein-based COVID-19 vaccine developed using an innovative approach to traditional vaccine technology.”
“Consistent with submissions to regulatory authorities worldwide, we have already submitted an amendment with updated manufacturing information for the EUA to the FDA for review. We look forward to collaborating with the FDA as it makes its final decision,” Erck added.
Prior Approvals
Over 40 countries have authorized the use of the Novavax COVID-19 vaccine in individuals aged 18 and above. The vaccine has also been granted Emergency Use Listing (EUL) by the World Health Organization (WHO).
Nevertheless, the U.S. has not authorized the Novavax COVID-19 vaccine for use in the nation.
Wall Street’s Take
Prior to the recent recommendation for the Novavax COVID-19 vaccine, Cowen & Co. analyst Georgi Yordanov reiterated a Buy rating and a price target of $150 (215.52% upside potential) on the stock.
Yordanov said, “As for the near-term impact of the potential EUA approval, recall, as part of the OWS funding, Novavax has already committed to supplying the U.S. government with 100MM+ doses of Nuvaxovid, and, therefore, we do not expect that it will have any meaningful near-term revenue implications.”
“However, we do believe this potential approval will remove a major overhang for the stock as it would finally allow Novavax to establish their presence on the U.S. market,” the analyst added.
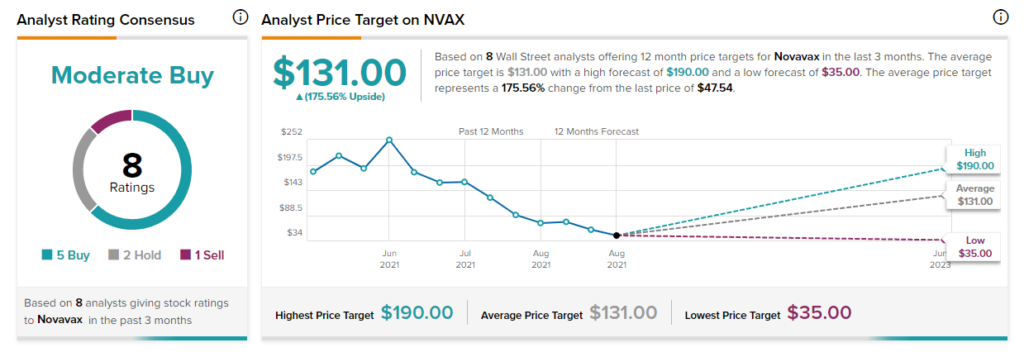
Looking at the consensus breakdown, based on five Buys, two Holds, and one Sell, the rest of the Street is cautiously optimistic about the stock, with a Moderate Buy rating. The average Novavax price target of $131 implies 175.56% upside potential. Shares have lost more than 76% over the past year.
Risk Analysis
According to the new TipRanks’ Risk Factors tool, Novavax stock is at risk mainly from three factors: Tech and Innovation, Legal and Regulatory, and Finance and Corporate, which contribute 27%, 27%, and 19%, respectively, to the total 62 risks identified for the stock.
Given the already high-risk profile of the company, investors may be cautious before investing in this stock.

Bottom-Line
The positive recommendation for the Novavax COVID-19 vaccine in adults is a significant step toward gaining approval in the United States. With the spread of many Omicron sub-variants leading to an upsurge in cases across the country, the protein-based vaccine will provide an alternative for individuals who are not in favor of mRNA vaccines.
Read full Disclosure









