Mylan Inc. (MYL) announced on Monday that remdesivir, the experimental drug candidate developed by Gilead Sciences Inc. (GILD), has been granted emergency use in COVID-19 patients by India’s regulator.
Mylan shares are advancing 5.2% to $16.75 and Gilead is up 2% at $77.90 in Monday’s pre-market trading.
The drugmaker, which in May signed a licensing agreement with Gilead for the commercialization of remdesivir in 127 low- and middle-income countries, said that the Drug Controller General of India (DCGI) has approved remdesivir 100 mg/vial for restricted emergency use in India as part of the regulator’s accelerated approval process to address urgent, unmet needs during the pandemic.
The antiviral drug has been approved for the treatment of suspected or laboratory confirmed incidences of COVID-19 in adults and children hospitalized with severe disease symptoms. Remdesivir will be launched under the brand name DESREM in India, and will be made available to patients in July at a price of INR 4,800 ($64.33), which is more than 80% less than the price at which it will be available to governments in the developed world.
“Mylan and Gilead have partnered for many years to make high quality medicines and have made significant progress to reduce the incidence of infectious diseases, including HIV/AIDS, around the world,” Mylan President Rajiv Malik said. “Our approval is a significant milestone for Mylan, for the global public health community and, most importantly, for patients who are battling this pandemic.”
Mylan will manufacture remdesivir in India at its injectables facilities, which also make products for the U.S. and have been inspected by the U.S. Food and Drug Administration (FDA) for compliance.
The drugmaker added that it continues to work extensively toward expanding emergency use access for patients in the 127 low- and middle-income countries where it is licensed by Gilead to do so. The approval by DCGI in India represents the first for Mylan in these 127 markets.
Remdesivir is a viral RNA polymerase inhibitor which means that it interferes with the production of viral genetic material, preventing the virus from multiplying. The antiviral therapy has just received conditional marketing authorization by the European Commission. The drug has been granted Emergency Use Authorization (EUA) by the U.S Food and Drug Administration to treat COVID-19 and has also been approved as a treatment for COVID-19 patients in Japan, Taiwan, Singapore, and the United Arab Emirates.
Gilead last week priced remdesivir at $2,340 per patient for a 5-day treatment in the U.S. and other developed countries.
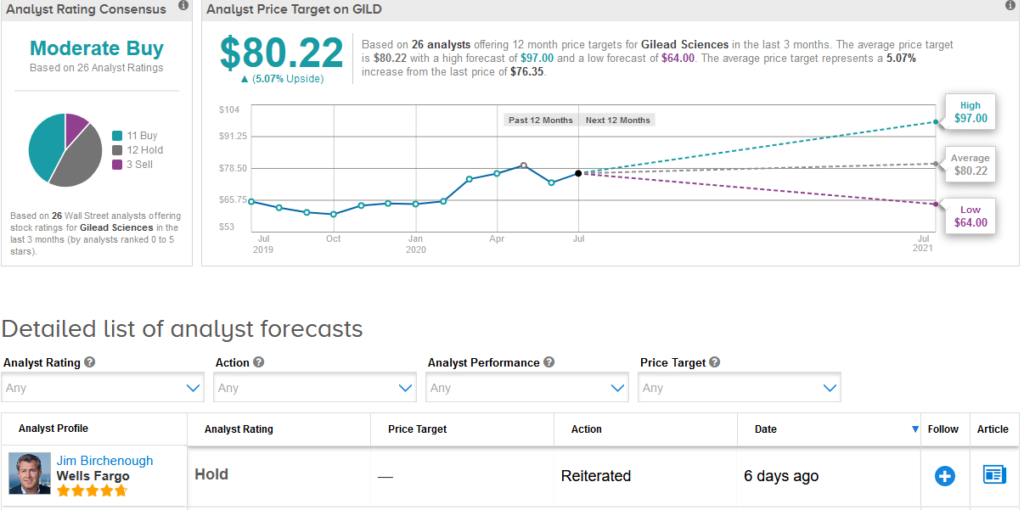
Five-star analyst Jim Birchenough at Wells Fargo reiterated a Hold rating on the stock saying that although pricing is below the high end of recommendations and represents “a responsible public health decision”, he remains skeptical on the longer-term commercial opportunity in view of emerging competition and potential vaccine progress.
The rest of the Street is cautiously optimistic on the stock. The Moderate Buy analyst consensus is based on 11 Buy ratings versus 12 Hold ratings and 3 Sell ratings.
Shares in Gilead have increased 18% this year and analysts have a $80.22 average price target implying 5.1% upside potential in the shares. (See Gilead stock analysis on TipRanks).
Related News:
CytoDyn Signs Distribution Deal For Covid-19 Treatment Leronlimab
Gilead’s Covid-19 Remdesivir Therapy Gets Conditional European Nod
Sanofi, GSK Close To $624M Covid-19 Vaccine Deal With U.K. – Report









