Acadia Pharmaceuticals (NAZDAQ: ACAD) has whipsawed recently over concerns regarding its planned resubmission of a supplemental New Drug Application (sNDA) for its flagship therapeutic, NUPLAZID. Its flagship intervention, NUPLAZID (pimavanserin), was approved in the U.S. by the FDA in April 2016 for the treatment of hallucinations and delusions associated with Parkinson’s disease psychosis, and Acadia has since pushed for an expansion of the label for the treatment of hallucinations and delusions associated with dementia-related psychosis.
Meet Your ETF AI Analyst
- Discover how TipRanks' ETF AI Analyst can help you make smarter investment decisions
- Explore ETFs TipRanks' users love and see what insights the ETF AI Analyst reveals about the ones you follow.
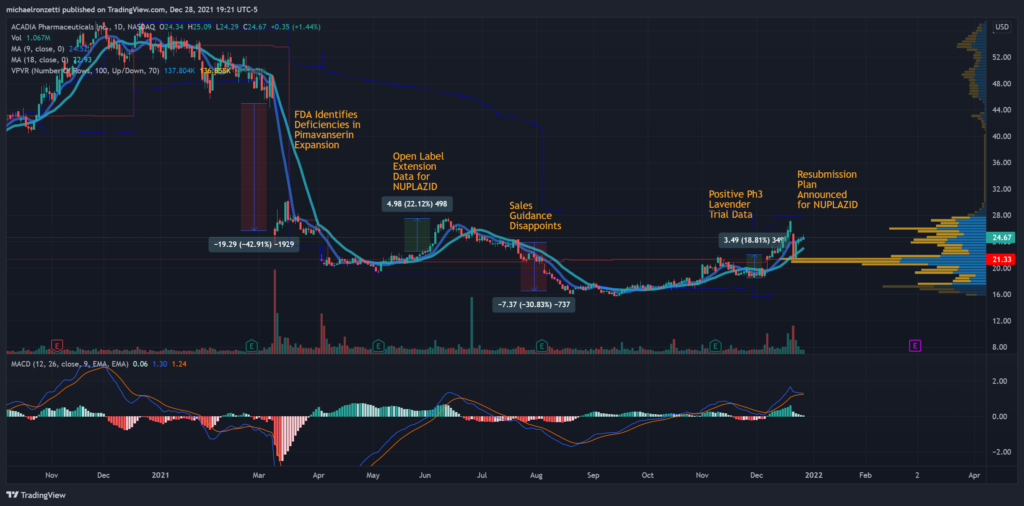
Unexpectedly, that process hit a major speedbump this past March, when the FDA “identified deficiencies” in the company’s dataset, spooking investors and analysts into a 43% dump of value and leaving this author with a neutral sentiment on the stock.
A Quick Look at Acadia
Acadia Pharmaceuticals is a midcap neuroscience biotech with a single approved drug, NUPLAZID (pimavanserin), for which the company has been working diligently to expand indications. Currently, NUPLAZID is its only marketed drug, approved for Parkinson’s disease psychosis and bringing in revenue of $131.6 million in 21Q3 (up 9% year-over-year).
On December 20, 2021, Acadia announced a planned resubmission in 22Q1 of the NUPLAZID sNDA for the treatment of hallucinations and dementia in a more targeted Alzheimer’s disease psychosis subgroup. Although the FDA has said (in previous guidance) that a new randomized controlled trial would be the strongest data, the FDA even agreeing to review additional analyses and arguments is an encouraging sign. Steve Davis, CEO, had the following to say on the resubmission matter:
“Following our recent meetings with the FDA, we plan to resubmit our sNDA for pimavanserin, narrowing the proposed indication from dementia-related psychosis to Alzheimer’s disease psychosis. Our resubmission will include new analyses of existing clinical study data supporting the treatment of hallucinations and delusions associated with Alzheimer’s disease. ”
Steve Davis, Chief Executive Officer.
Analyst Sentiment
Still, analysts are still digesting the resubmission news, but of the 11 analysts that have put out guidance with ACAD price targets since this resubmission news, three have given Buy ratings while eight have given it a Hold. Taken together, the analysts present an average price target of $29.54, representing 23.3% upside growth.
However, when we split the targets by rating, the “Buy” ratings have an average price target of 36.0 (+/- 5.3), representing about a 50.25% upside, while the “Hold” ratings only present an average price target of 27.1 (+/- 5.3), representing only 13.1% upside. This clear split in analyst sentiment can most likely be attributed to the heavy weight placed on this binary FDA resubmission event.

Although there’s no clear guidance on whether or not the FDA will approve this indication expansion with only ad-hoc analysis, those with bullish sentiment on the binary event are not without cause for hope. Recent data in the HARMONY study on pimavanserin provides key momentum towards approval for a narrowed Alzheimer’s disease subset, and the study lead’s author had a very positive outlook, saying,
“The relapse prevention design of the HARMONY study mirrors exactly what we do in clinical practice. This landmark trial showed that when patients responded to pimavanserin and then continued treatment, they were almost three times less likely to develop recurrence of their hallucinations and delusions than those patients who discontinued pimavanserin treatment. This is a substantial finding and a significant advance for a critical public health need in our field. There is no FDA approved treatment for DRP, and the majority of antipsychotics currently used off-label have equivocal efficacy and may accelerate cognitive decline.”
Dr. Pierre N. Tariot, Banner Alzheimer’s Institute director, HARMONY Study Lead
There are plenty of other catalysts for volatility with Acadia coming in the first quarter of the new year, including:
- 40th J.P. Morgan Healthcare Conference on Tuesday, January 11, 2022 (10:30 a.m. EST).
- Phase 2 ACP-044 data release
- Earnings disclosure on February (Feb 28, 2022)
- NDA submission for trofinetide (Rett Syndrome)
Final Technical Evaluation and Summary
As usual, I’ll present a brief technical evaluation of the security in the intermediate term. The most obvious signal is the large 43% gap from the negative FDA letter regarding the Pimavanserin expansion. Since then, positive extension data for the treatment has provided bumps in the price (+22% in June, +18% in November), and the sNDA for NUPLAZID remains the largest near-term catalyst.
Acadia has been trading off a bounce in VWAP and on a bullish 9/18 day simple moving average crossover. The stock is still in a bullish MACD signal on higher-than-average volume, relaying a bullish sentiment on the equity.
Disclosure: At the time of publication, Michael Ronzetti did not have a position in any of the securities mentioned in this article.
Disclaimer: The information contained in this article represents the views and opinion of the writer only, and not the views or opinion of TipRanks or its affiliates. Read full disclaimer >>>
















