Shares of Sarepta Therapeutics (SRPT) plunged after the company reported disappointing results from a key trial of its Duchenne muscular dystrophy (DMD) therapy. The data missed investor expectations, raising fresh concerns about the drug’s long-term potential and Sarepta’s overall growth outlook. The company released the findings alongside its Q3 2025 results, sending SRPT stock down more than 37% in pre-market trading on Tuesday.
Elevate Your Investing Strategy:
- Take advantage of TipRanks Premium at 50% off! Unlock powerful investing tools, advanced data, and expert analyst insights to help you invest with confidence.
For context, Sarepta is focused on developing genetic medicines to treat rare neuromuscular diseases, including Duchenne muscular dystrophy (DMD). Meanwhile, DMD is a genetic disorder that causes progressive muscle weakness and loss.
Duchenne Trial Falls Short
The study, which took nine years to complete, included 225 boys aged 6 to 13 diagnosed with DMD. The trial tested two drugs, casimersen and golodirsen, which belong to a class of medicines called PMOs (phosphorodiamidate morpholino oligomers). These drugs are designed to help patients produce functional dystrophin, a protein crucial for muscle strength.
After 96 weeks, patients showed some improvement in their ability to climb four steps, but the results were not statistically significant. Sarepta reported a small difference of 0.05 steps per second on the main study measure.
The company noted that COVID-19 disruptions affected participation and data collection, which may have influenced the results. Still, Sarepta highlighted that, when excluding patients impacted by the pandemic, the drugs appeared to slow disease progression by about 30%, with long-term data suggesting a three-year delay in wheelchair dependence.
Is There Still Hope for Sarepta?
Despite the setback, Sarepta said it plans to meet with the U.S. Food and Drug Administration (FDA) to discuss turning its drugs’ accelerated approvals into full approvals. The company’s executives emphasized that they don’t believe there’s a risk of losing marketing authorization, citing the drugs’ strong safety profile.
Notably, J.P. Morgan analyst Anupam Rama remains optimistic, noting that the trial’s miss could be explained by COVID-related disruptions. He also highlighted that results excluding affected patients showed encouraging trends.
Rama added that while there’s a solid case for full approval, the regulatory process remains unpredictable in the current environment.
Sarepta Reports Q3 Results
On a positive note, Sarepta reported strong third-quarter 2025 results, beating Wall Street estimates by a wide margin. The company posted an EPS loss of $0.13, far better than the expected loss of $0.70.
Moreover, revenue came in at $399 million, up 18% from analysts’ forecast of $337.9 million.
Is SRPT Stock a Good Buy Now?
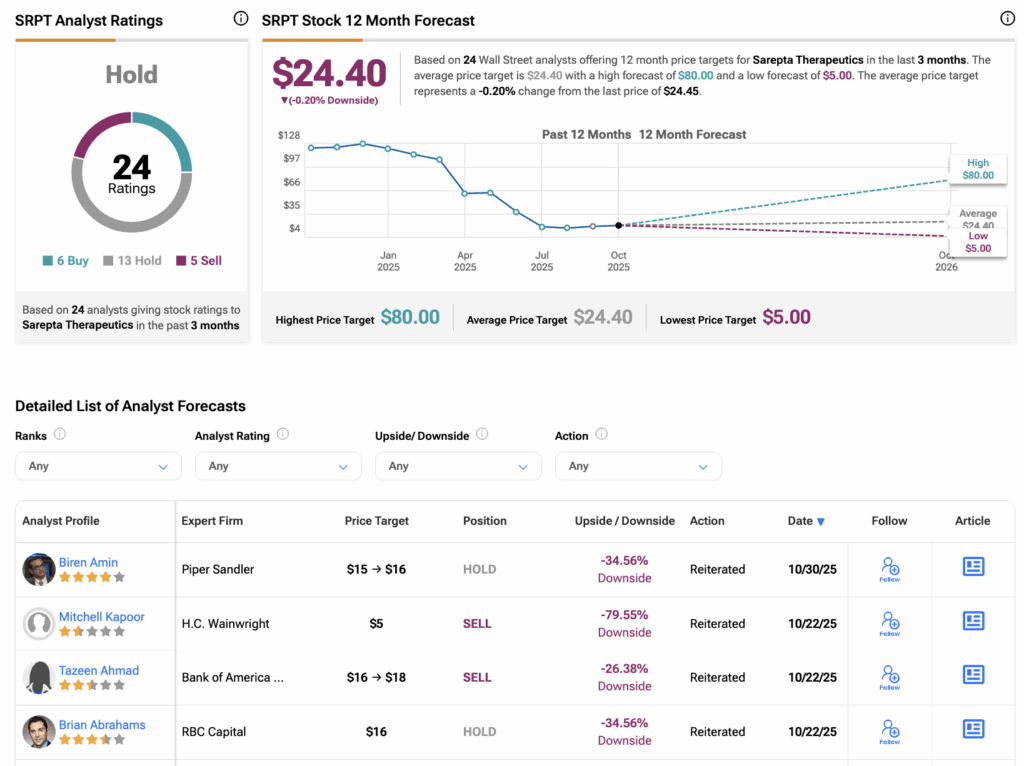
According to TipRanks, Wall Street has a Hold consensus rating on SRPT stock, based on six Buys, 13 Holds, and five Sells assigned in the last three months. The average Sarepta Therapeutics stock price target of $24.40 implies a downside of 0.20% from the current level.