Shares of biopharmaceutical company Viridian Therapeutics (NASDAQ:VRDN) are tanking in the pre-market session today after it announced positive preliminary data from a Phase 1/2 study evaluating VRDN-001 for the treatment of chronic thyroid eye disease.
Elevate Your Investing Strategy:
- Take advantage of TipRanks Premium at 55% off! Unlock powerful investing tools, advanced data, and expert analyst insights to help you invest with confidence.
The drug was observed to be well tolerated in subjects and at only two infusions (dosing of 10 mg/kg and 3 mg/kg) demonstrated a decrease in proptosis alongside an improved clinical activity score in patients. Further, there were no reported serious adverse events in the trial.
Additionally, after discussions with the U.S. Food and Drug Administration (FDA), Viridian is amending the design of THRIVE Phase 3 to include a five-dose regimen and placebo arms only. The change comes after stakeholder feedback pointed to a preference for a shorter dose regimen.
Next, topline results from Thrive Phase 3 are anticipated in the middle of next year and from THRIVE-2 Phase 3 by the end of next year.
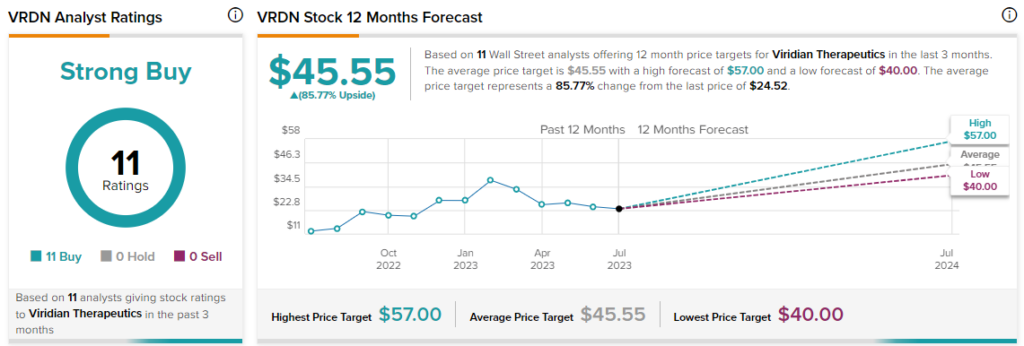
Overall, the Street has a $45.55 consensus price target on VRDN alongside a Strong Buy consensus rating. Short interest in the stock currently stands at nearly 17.2%.
Read full Disclosure
















