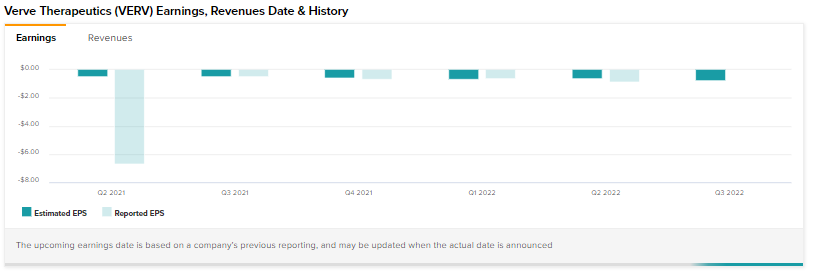
Shares of gene editing technology company Verve Therapeutics (NASDAQ:VERV) are tanking today after reporting a wider-than-expected third-quarter loss.
Elevate Your Investing Strategy:
- Take advantage of TipRanks Premium at 50% off! Unlock powerful investing tools, advanced data, and expert analyst insights to help you invest with confidence.
The top line came in at $0.93 million, ahead of expectations by $0.1 million. Net loss per share at $0.79 though came in wider than estimates by $0.06. The company had a cash pile of $550.7 million at the end of September.
Separately, the U.S. Food and Drug Administration placed a hold on Verve’s IND application for its product candidate VERVE-101.
The company had requested clearance to conduct a clinical trial of the drug for the treatment of heterozygous familial hypercholesterolemia.
Shares of the company are down nearly 20% today as a result.
Read full Disclosure
















