Shares of gene therapy company uniQure (NASDAQ:QURE) are down nearly 38% at the time of writing today after it announced interim data (up to 24 months of follow-up) from a Phase 1/2 study evaluating AMT-130 for the treatment of Huntington’s disease.
Elevate Your Investing Strategy:
- Take advantage of TipRanks Premium at 50% off! Unlock powerful investing tools, advanced data, and expert analyst insights to help you invest with confidence.
The drug was observed to be generally well-tolerated with evidence of clinical and functional benefits. While a total of four serious adverse events have been reported so far, after reviewing interim data analysis, the Data Safety Monitoring Board has recommended continuing the development of the drug.
Importantly, the drug has demonstrated benefits across the total motor score, and functional capacity as well as a mean 0.9 improvement in the composite unified Huntington’s disease rating scale at a low dose and a mean 1.0 improvement at a high dose.
Next, the company expects to complete enrollment in a high-dose cohort in Europe in early Q3 2023 with the initiation of a third cohort in the U.S. anticipated in H2 2023. Additionally, new clinical data from the Phase 1/2 study is awaited in Q4 2023 with regulatory interactions in the U.S. as well as the E.U. expected by Q1 2024.
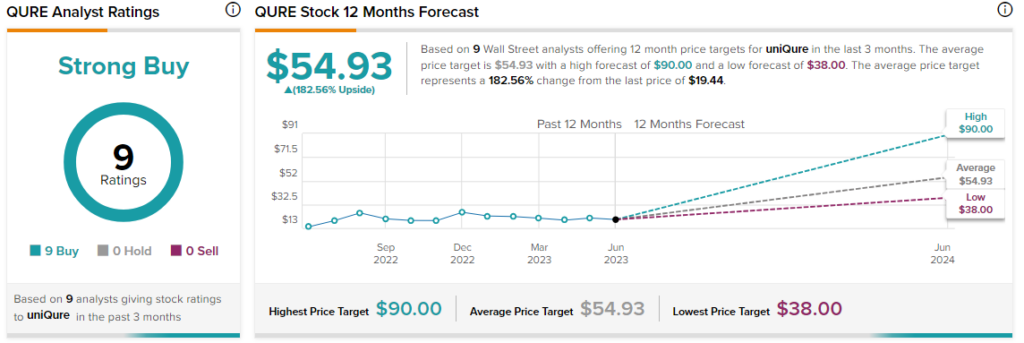
Overall, the Street has a $54.93 consensus price target on uniQure alongside a Strong Buy consensus rating. Despite today’s price decline, shares of the company are still up about 28% over the past year.
Read full Disclosure
















