Tiziana Life Sciences (NASDAQ:TLSA) shares jumped by over 45% at the time of writing after the U.S. FDA gave a thumbs-up for the Investigational New Drug application for foralumab. This nod opens the door for studies on the drug’s efficacy as an intranasal treatment for Alzheimer’s. The UK biotech firm plans to put foralumab under the microscope both on its own and alongside another FDA-endorsed therapy, aiming to combat the neuroinflammation characteristic of Alzheimer’s.
Elevate Your Investing Strategy:
- Take advantage of TipRanks Premium at 55% off! Unlock powerful investing tools, advanced data, and expert analyst insights to help you invest with confidence.
CEO Gabriele Cerrone couldn’t help but express his enthusiasm, dubbing the FDA’s green light as a monumental validation of foralumab’s promise. Nevertheless, Alzheimer’s isn’t their sole focus—come Q3 2023, the company’s gearing up to initiate a Phase 2 trial, casting a spotlight on how foralumab fares against a specific form of multiple sclerosis.
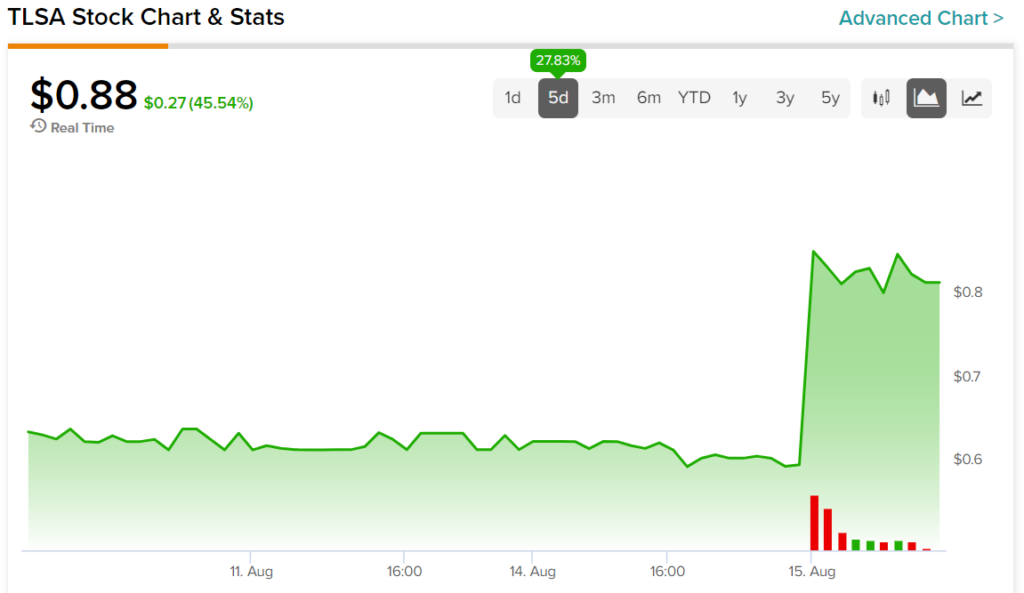
A look at the past five trading days for TLSA stock highlights the level of impact today’s news had on it. Indeed, investors are now up 27.83% during this timeframe.
















