Shares of medical aesthetics company Sientra, Inc. (NASDAQ:SIEN) are up nearly 74% in the pre-market session today after the U.S. Food and Drug Administration provided a 510k-clearance for its AlloX2 Pro Tissue Expander.
Pick the best stocks and maximize your portfolio:
- Discover top-rated stocks from highly ranked analysts with Analyst Top Stocks!
- Easily identify outperforming stocks and invest smarter with Top Smart Score Stocks
The AlloX2 Pro removes 95% of the metal associated with tissue expander ports which makes it the only tissue expander with clearance for exposure to magnetic resonance imaging in the U.S. market!
Moreover, the product also offers minimal interference with radiation therapy in post-mastectomy patients, rapid port filling and drainage as well as a softer drain ensuring a comfortable experience.
Impressively, this is the third new product clearance for the company in the last 12 months and its share in the breast reconstruction market has already reached 23% at the end of the first quarter.
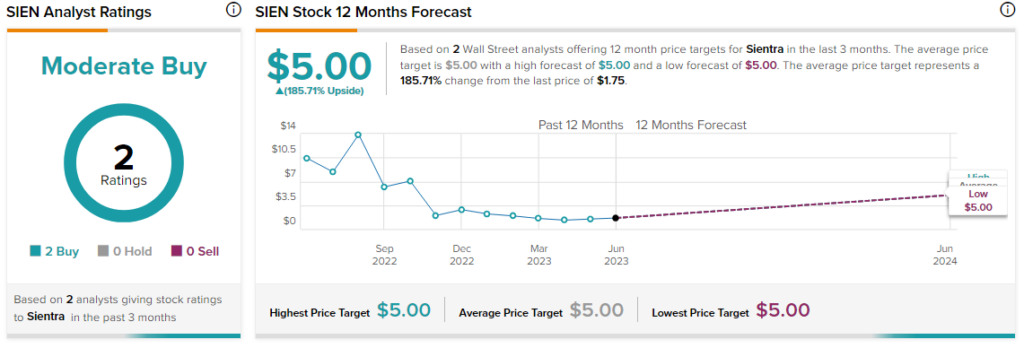
Overall, the Street has a $5 consensus price target on SIEN alongside a Moderate Buy consensus rating. This points to a massive 185.7% potential upside in the stock.
Read full Disclosure



















