The trading of Sarepta Therapeutics (NASDAQ:SRPT) shares has hit the pause button, as an independent expert group from the FDA is convening on Friday to discuss the company’s application to market its Duchenne muscular dystrophy targeted gene therapy, SRP-9001. The FDA’s Cellular, Tissue, and Gene Therapies Advisory Committee members will cast a vote by the end of the meeting to decide on the therapy’s approval.
Meet Your ETF AI Analyst
- Discover how TipRanks' ETF AI Analyst can help you make smarter investment decisions
- Explore ETFs TipRanks' users love and see what insights the ETF AI Analyst reveals about the ones you follow.
Given the high stakes, several investment firms have been trying their hand at predicting the meeting’s outcome. Analyst Neena Bitritto-Garg, noting the brief two-hour discussion window before voting, anticipates a one-sided decision. Meanwhile, SVB Securities analyst Joseph Schwartz warns that the FDA’s briefing documents create an uncertain atmosphere for the panel members, and Evercore ISI analysts are betting on a 50%-60% approval chance.
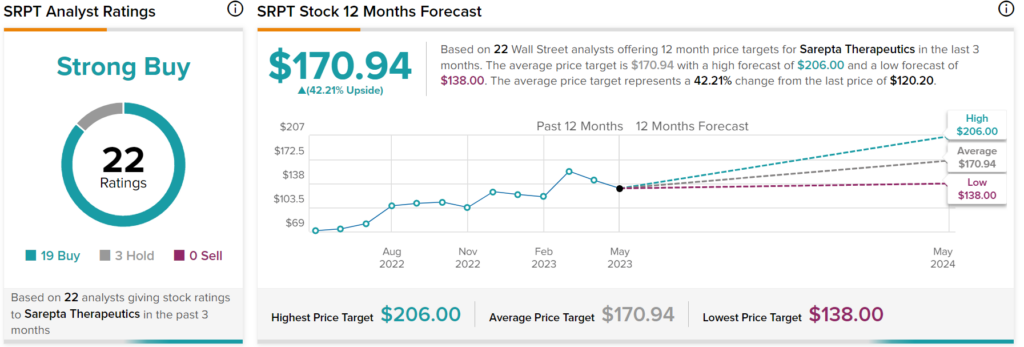
Overall, Wall Street analysts have a consensus price target of $170.94 on SRPT stock, implying over 42% upside potential, as indicated by the graphic above.
















