Shares of Sarepta Therapeutics (NASDAQ: SRPT) slid more than 20% in pre-market trading on Friday after the medical research and drug development company announced that the U.S. Food and Drug Administration (FDA) will set up an advisory committee meeting which will be held for SRP-9001
in advance of the May 29 regulatory action date. SRP-9001 is the company’s investigational gene therapy for the treatment of Duchenne muscular dystrophy.
Meet Your ETF AI Analyst
- Discover how TipRanks' ETF AI Analyst can help you make smarter investment decisions
- Explore ETFs TipRanks' users love and see what insights the ETF AI Analyst reveals about the ones you follow.
The FDA meeting will be in relation to Sarepta’s biologics license application (BLA) for SRP-9001.
Doug Ingram, President, and CEO, of Sarepta commented, ” FDA’s decision to hold a public advisory committee meeting on the SRP 9001 BLA is a change from the communicated position at the midcycle meeting. It is our understanding that as one of the first gene therapy BLAs founded on a surrogate endpoint, the advisory committee will primarily relate to the totality of evidence supporting the conclusion that the SRP 9001 dystrophin is reasonably likely to predict clinical benefit, the standard for accelerated approval.”
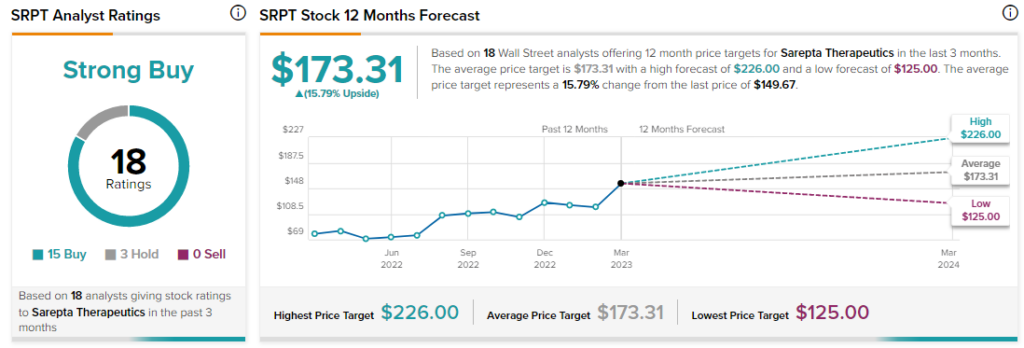
Analysts, however, remain bullish about SRPT stock with a Strong Buy consensus rating based on 15 Buys and three Holds.
















