Sage Therapeutics (NASDAQ:SAGE) shares are down a massive 50% at the time of writing after the U.S. Food and Drug Administration (FDA) approved its product candidate zuranolone in postpartum depression but refused approval in major depressive disorder (MDD) owing to lack of efficacy.
Elevate Your Investing Strategy:
- Take advantage of TipRanks Premium at 50% off! Unlock powerful investing tools, advanced data, and expert analyst insights to help you invest with confidence.
While zuranolone has now become the first and only approved oral treatment for postpartum depression, investor sentiment in the stock is taking a beating today as the complete response letter (CRL) from the FDA for the drug in MDD has pointed to a requirement for additional study or studies for potential approval.
Additionally, the biopharmaceutical company also announced second-quarter numbers with both its top line and bottom line falling short of the Street’s expectations. Revenue rose 64.8% year-over-year to $2.47 million but missed the cut by $0.44 million. Net loss per share at $2.68 too came in wider than expectations by $0.13.
Next, Sage is planning to ‘thoroughly review’ the feedback from the healthcare regulator and is aiming to launch zuranolone in PPD in the fourth quarter. It had a cash pile of $1 billion at the end of June 2023 but after the CRL, is looking at pipeline prioritization and workforce reorganization to extend its cash runway.
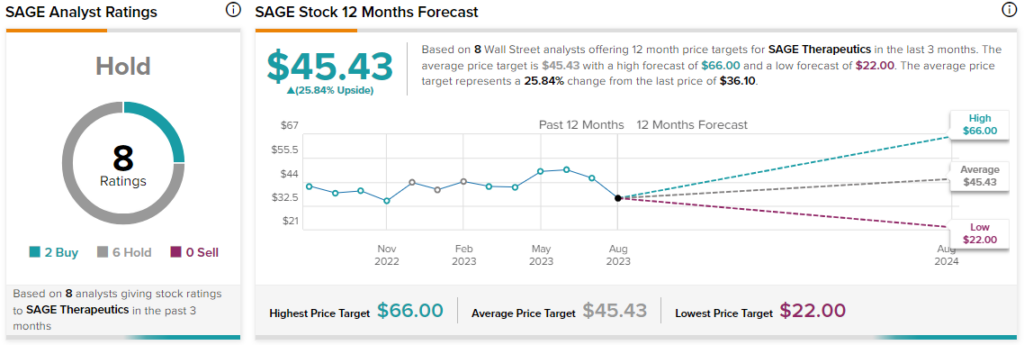
Overall, the Street has a $45.43 consensus price target on Sage alongside a Hold consensus rating. With today’s price erosion Sage shares have now dropped nearly 60% over the past month alone.
Read full Disclosure
















