Late-stage biotech company, Rocket Pharmaceuticals (NASDAQ: RCKT) surged in pre-market trading on Wednesday after the company announced that it had reached an “alignment” with the U.S. Food and Drug Administration (FDA) regarding a global Phase 2 pivotal trial of RP-A501 for Danon Disease.
Danon Disease is a fatal inherited cardiomyopathy that leads to mortality in the majority of male patients at the age of around 20 years and females at age 40 years. According to the company, Danon disease affects approximately 15,000 to 30,000 patients in the U.S. and Europe.
This will be a global single-arm, multi-center Phase 2 pivotal trial that will look at the efficacy and safety of RP-A501 in 12 patients with Danon Disease.
Rocket Public Share Offering
In another development, Rocket announced the pricing of its public offering of 7.8 million shares of its common stock at a price of $16 per share and will allow certain investors with pre-funded warrants to purchase 3.12 million shares at a price of $15.99 per pre-funded warrant, which indicates the “per share public offering price for the common stock less the $0.01 per share exercise price for each such pre-funded warrant.”
The company expects to have gross proceeds of around $175 million from the public offering. In addition, Rocket has granted underwriters a 30-day option to purchase up to an additional 1.64 million shares of its common stock. The offering is expected to close on or about September 15, subject to customary closing conditions.
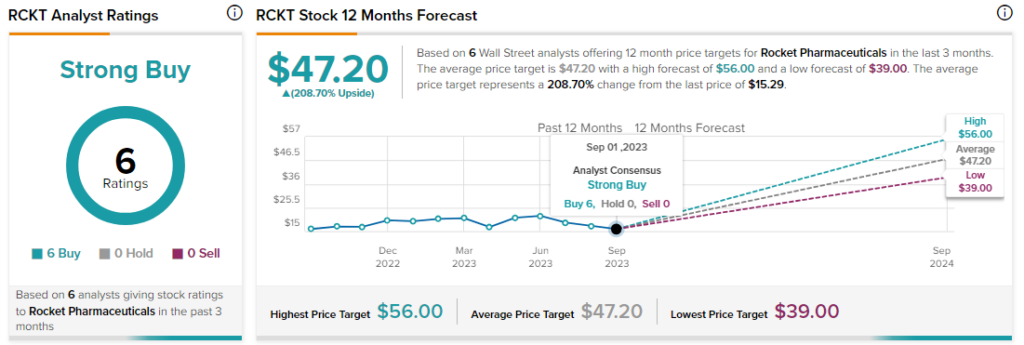
Analysts remain bullish about RCKT stock with a Strong Buy consensus rating based on a unanimous six Buys.
















