Shares of diagnostics solutions company Renalytix (NASDAQ:RNLX) are up a massive 40% at the time of writing today after its KidneyIntelX.dkd prognostic test bagged a De Novo marketing approval from the U.S. Food and Drug Administration (FDA).
Elevate Your Investing Strategy:
- Take advantage of TipRanks Premium at 50% off! Unlock powerful investing tools, advanced data, and expert analyst insights to help you invest with confidence.
With this approval, the AI-enabled testing platform can now help guide care management in type 2 diabetes and early-stage kidney disease (diabetic).
Importantly, the authorization can mean higher test adoption, an expansion in insurance coverage as well as further international approvals for KidneyIntelX.dkd. The test categorizes patients into three risk levels (low, moderate, and high) and provides comprehensive data on the risk of progressive decline in kidney function.
Impressively, as a laboratory-developed test, KidneyIntelX has already delivered results in nearly 10,000 patients in the U.S. Kidney disease impacts more than 850 million people worldwide and ~15% of adults in the U.S. are estimated to suffer from chronic kidney disease according to the Centers for Disease Control and Prevention (CDC).
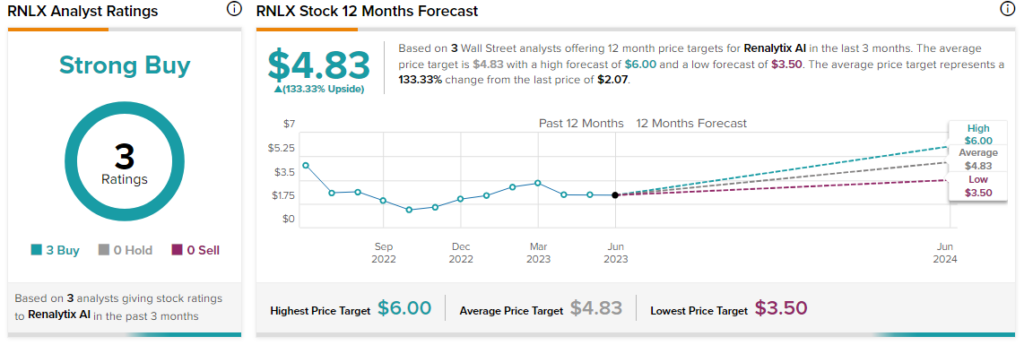
Overall, the Street has a $4.83 consensus price target on Renalytix alongside a Strong Buy consensus rating. Today’s price surge comes after a nearly 49.6% drop in shares of the company over the past year.
Read full Disclosure