Shares of biopharmaceutical company Reata Pharmaceuticals (NASDAQ:RETA) have more than doubled in the pre-market session today after the U.S. Food and Drug Administration approved Skyclarys for the treatment of Friedreich’s Ataxia.
Pick the best stocks and maximize your portfolio:
- Discover top-rated stocks from highly ranked analysts with Analyst Top Stocks!
- Easily identify outperforming stocks and invest smarter with Top Smart Score Stocks
Along with the approval, the company has also bagged a rare pediatric disease priority voucher from the regulator. With this approval, Skyclarys becomes the first therapy on the market targeted at Friedreich’s Ataxia, which is an ultra-rare, inherited neurodegenerative disorder. The condition affects about 5,000 patients in the U.S.
Next, the company is completing commercial drug production and expects it to be commercially available in the second quarter.
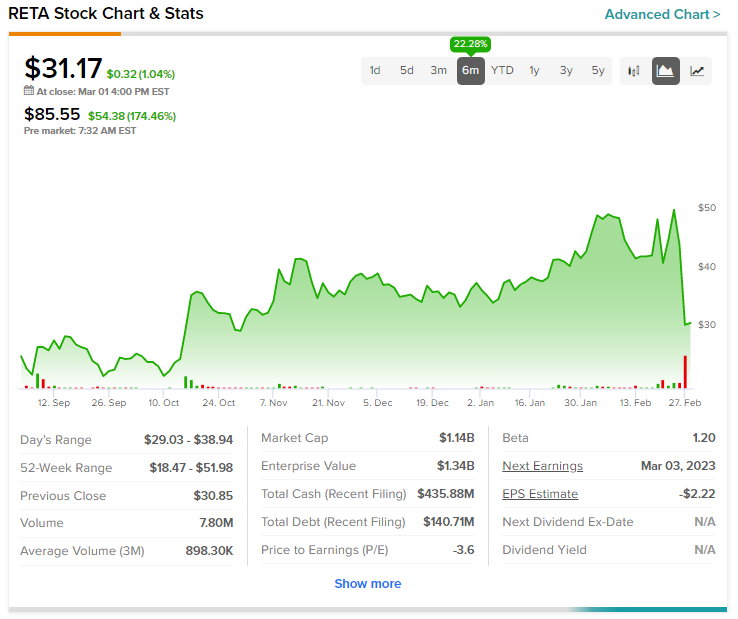
Shares of the company had inched up 29.5% over the past six months and are up a further nearly 170% today already.
Read full Disclosure



















