Shares of Pieris Pharmaceuticals (NASDAQ:PIRS), a biotech company focusing on innovative treatments for lung diseases and cancer, cratered today after announcing that its partner, AstraZeneca (NASDAQ:AZN), has decided to halt the ongoing clinical trials for elarekibep, a drug being developed for asthma treatment. The decision came after a 13-week non-human trial revealed lung damage that, while not impacting the current clinical studies, could hinder long-term use and further development of the drug. This decision from AstraZeneca was independent of any data from the ongoing Phase 2a human trial. Pieris will now closely review the fallout from these findings and the subsequent decision, adjusting their company goals as necessary before providing a further update.
Meet Your ETF AI Analyst
- Discover how TipRanks' ETF AI Analyst can help you make smarter investment decisions
- Explore ETFs TipRanks' users love and see what insights the ETF AI Analyst reveals about the ones you follow.
The non-human study was conducted on primates over 13 weeks and included three different dosage groups. Although no clinical observations were made across the varying dosages, lung tissue damage caused by inflammation was found. Commenting on these disappointing results, Pieris’ CEO, Stephen Yoder, explained that for the drug to move into later stages of development, it needs to show that it can be safely used long-term, something the recent toxicology data does not support. In light of these results, Pieris will be reevaluating its plans and will share a corporate update after reviewing all possible options. The company acknowledged the expertise and resources AstraZeneca dedicated to the project and expressed gratitude to all physicians, patients, and caregivers involved in the study.
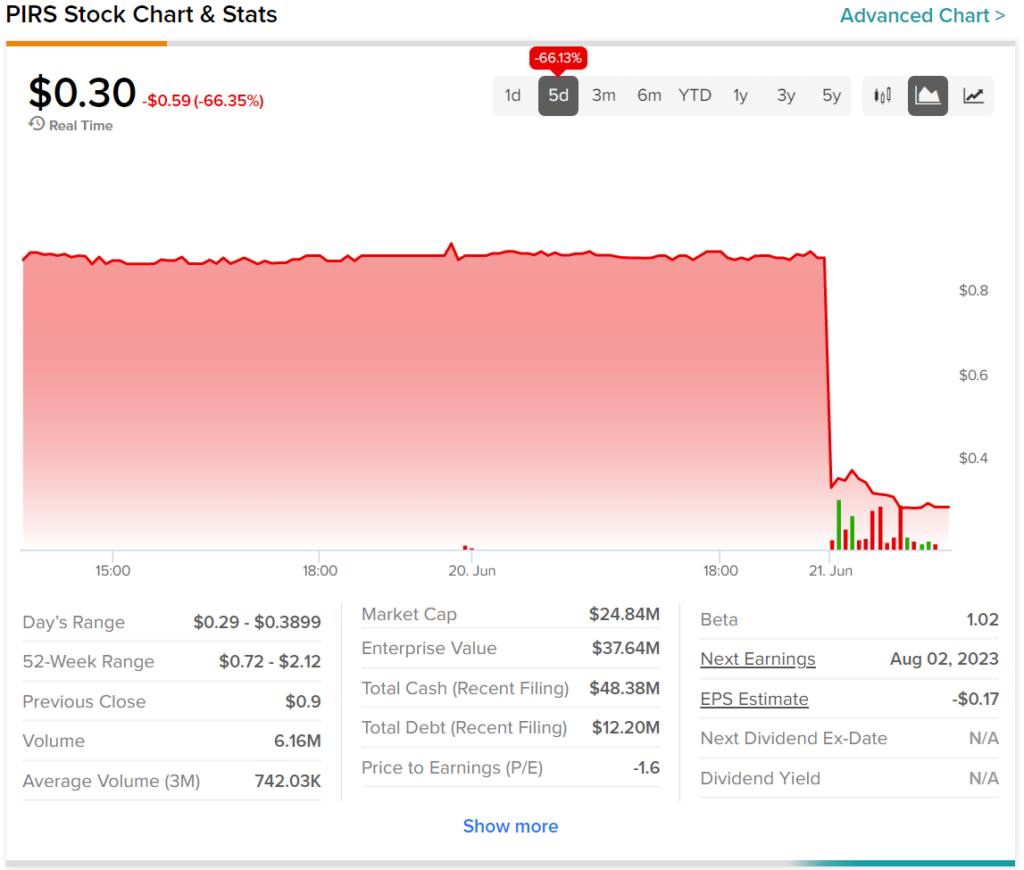
A look at the past five trading days for PIRS stock highlights the level of impact today’s news had on it. Indeed, shares fell over 66% at the time of writing. As a result, investors are now down 66.13% during this timeframe.
















