Biopharmaceutical company Outlook Therapeutics (NASDAQ: OTLK) cratered more than 83% on Wednesday. This happened after it announced that the U.S. Food and Drug Administration (FDA) issued a Complete Response Letter (CRL) to the company’s Biologics License Application (BLA) for ONS-5010. ONS-5010 is an investigational ophthalmic formulation of bevacizumab under development to treat wet age-related macular degeneration (AMD).
Elevate Your Investing Strategy:
- Take advantage of TipRanks Premium at 55% off! Unlock powerful investing tools, advanced data, and expert analyst insights to help you invest with confidence.
While the FDA acknowledged that the NORSE TWO pivotal trial met important safety and efficacy endpoints, it concluded that it “could not approve the BLA during this review cycle due to several CMC [chemistry, manufacturing, and controls] issues, open observations from pre-approval manufacturing inspections, and a lack of substantial evidence.”
Russell Trenary, President and CEO of Outlook Therapeutics, commented, “We will request a formal meeting as soon as possible with the FDA to further understand the BLA deficiencies and how best to resolve them. Following this meeting with the FDA, the Company will be able to discuss next steps and the expected timing for resolution.”
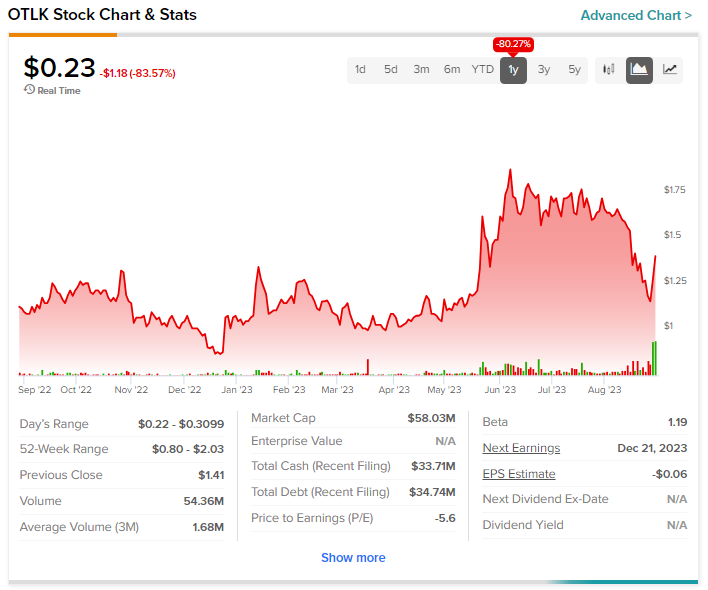
OTLK stock has not fared well in the past year and has tanked by more than 80%.
















