Shares of biopharmaceutical and diagnostics company Opko Health (NASDAQ:OPK) are up in double digits today after the U.S. Food and Drug Administration approved its growth hormone therapy Ngenla in pediatric patients aged three years and above.
Meet Your ETF AI Analyst
- Discover how TipRanks' ETF AI Analyst can help you make smarter investment decisions
- Explore ETFs TipRanks' users love and see what insights the ETF AI Analyst reveals about the ones you follow.
Opko collaborated with Pfizer (NYSE:PFE) for the development of Ngenla in 2014 and the approval comes after a Phase 3 trial demonstrated the non-inferiority of Ngenla versus somatropin. Growth hormone deficiency impacts one in 4,000 to 10,000 children and the once-a-week therapy can help lower the treatment burden of daily hormone injections.
Ngenla has already been approved for this indication in over 40 markets including Canada, Japan, and European Union member states.
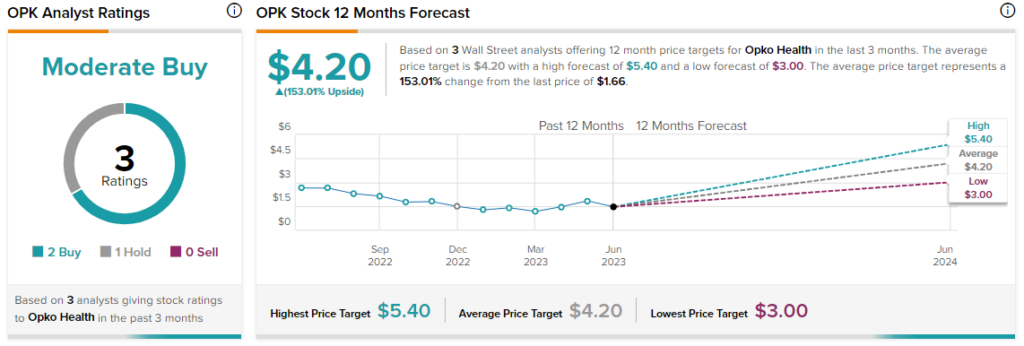
Overall, the Street has a $4.20 consensus price target on Opko Health alongside a Moderate Buy consensus rating. This points to a massive 153% potential upside in the stock on top of the 36% price surge year-to-date.
Read full Disclosure
















