The U.S. Food and Drug Administration (FDA) has extended the emergency use authorization (EUA) of a booster dose of Moderna, Inc.’s (MRNA) COVID-19 vaccine to all adults aged 18 and older. Following the news, shares of the American pharmaceutical and biotechnology company closed 4.9% higher on Friday.
Meet Your ETF AI Analyst
- Discover how TipRanks' ETF AI Analyst can help you make smarter investment decisions
- Explore ETFs TipRanks' users love and see what insights the ETF AI Analyst reveals about the ones you follow.
The dose provided by Moderna can be administered at the 50 µg level. Notably, the dose can be administered a minimum after six months of any primary COVID-19 vaccination.
The U.S. regulator’s authorization was based on the scientific evidence provided by the company, which included data analysis from the Phase 2 clinical study of mRNA-1273. Notably, the analysis was amended to offer a booster dose of mRNA-1273 at the 50 µg dose level to interested participants six to eight months following their second dose, the company said.
Last month, the company received Emergency Use Authorization (EUA) for a booster dose to people aged 65 and above, adults (aged 18 to 64) who are at a high risk of severe COVID-19, and people (aged 18 to 64) with frequent occupational exposure to SARS-CoV-2. Markedly, the booster vaccine has been authorized for adults aged 18 and above without any occupational risk factors in many international markets. This followed the earlier approval of the 100 µg mRNA 1273 third dose in immunocompromised individuals.
CEO Comments
The CEO of Moderna, Stephane Bancel, said, “This emergency use authorization comes at a critical time as we enter the winter months and face increasing COVID-19 case counts and hospitalizations across the country. We thank the FDA for their review, and are confident in the robust clinical evidence that a 50 µg booster dose of mRNA-1273 induces a strong immune response against COVID-19.”
See Top Smart Score Stocks on TipRanks >>
Other Development
Following the approval, the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) also recommended the booster dose of Moderna’s COVID-19 vaccine for people aged 18 and older.
Bancel said, “This is another important step in our quest to address this pandemic with our mRNA vaccine.” (See Moderna stock charts on TipRanks)
Wall Street’s Take
Following the FDA’s approval, Piper Sandler analyst Edward Tenthoff reiterated a Buy rating and a price target of $348 (31.9% upside potential) on the stock.
Tenthoff forecasts SpikeVax revenues of $17.5 billion in 2021 and $21 billion in 2022.
Overall, the stock has a Hold consensus rating based on 6 Buys, 4 Holds and 3 Sells. The average Moderna price target of $298.17 implies 13% upside potential from current levels. Shares have gained 60.7% over the past six months.
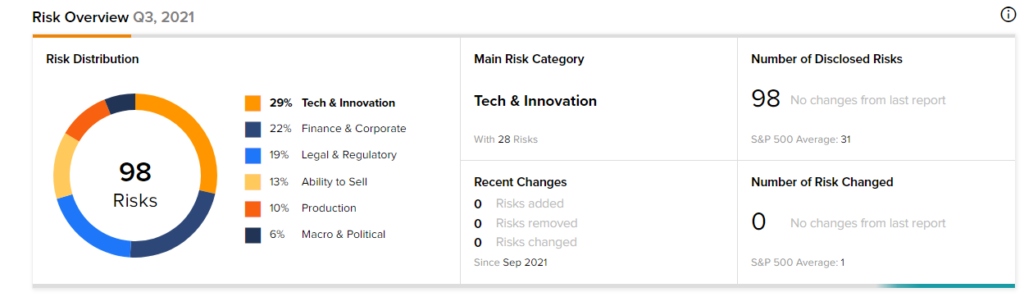
Risk Analysis
According to the new TipRanks’ Risk Factors tool, the Moderna stock is at risk mainly from three factors: Tech and Innovation, Finance and Corporate, and Legal and Regulatory, which contribute 29%, 22%, and 19%, respectively, to the total 98 risks identified for the stock.
Related News:
CVS Health to Commence New Retail Footprint Strategy with Closure of 900 Stores
NIKE Bumps Up Quarterly Dividend by 11%
Clover Health Prices Class A Common Stock Offering Worth $300M
















