Shares of Miromatrix (NASDAQ: MIRO) plunged more than 15% in after-hours trading on Wednesday after the life sciences company announced that the U.S. FDA had placed a clinical hold on its investigational New Drug (IND) application for Miroliverelap for the treatment of acute liver failure.
Meet Your ETF AI Analyst
- Discover how TipRanks' ETF AI Analyst can help you make smarter investment decisions
- Explore ETFs TipRanks' users love and see what insights the ETF AI Analyst reveals about the ones you follow.
The company submitted the IND application in mid-November.
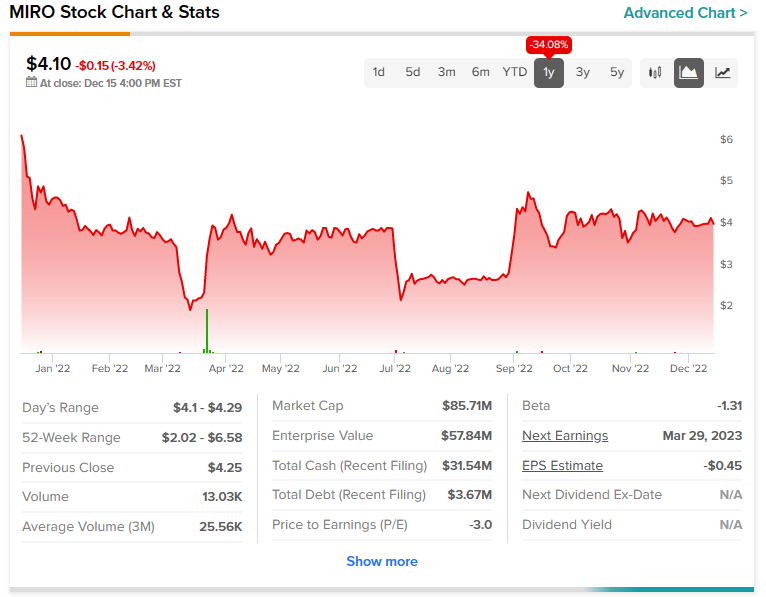
MIRO stock has plunged more than 34% in the past year.
















