Madrigal Pharmaceuticals (NASDAQ:MDGL) surged in pre-market trading after the company announced that the U.S. Food and Drug Administration (FDA) had granted accelerated approval for Rezdiffra (resmetirom). The U.S. FDA has approved the biopharmaceutical company’s Rezdiffra along with diet and exercise for the treatment of adults with non-cirrhotic NASH with moderate to advanced liver fibrosis. NASH is Noncirrhotic Nonalcoholic Steatohepatitis or fatty liver disease.
Elevate Your Investing Strategy:
- Take advantage of TipRanks Premium at 55% off! Unlock powerful investing tools, advanced data, and expert analyst insights to help you invest with confidence.
First Mover Advantage
Rezdiffra has become the first and only medication approved by the FDA for the treatment of NASH. This medication has gained accelerated approval based on Phase 3 data that showed improvement in liver fibrosis and resolution of NASH in noncirrhotic patients with moderate to advanced fibrosis. For prescribing Rezdiffra, no liver biopsy is required for diagnosis.
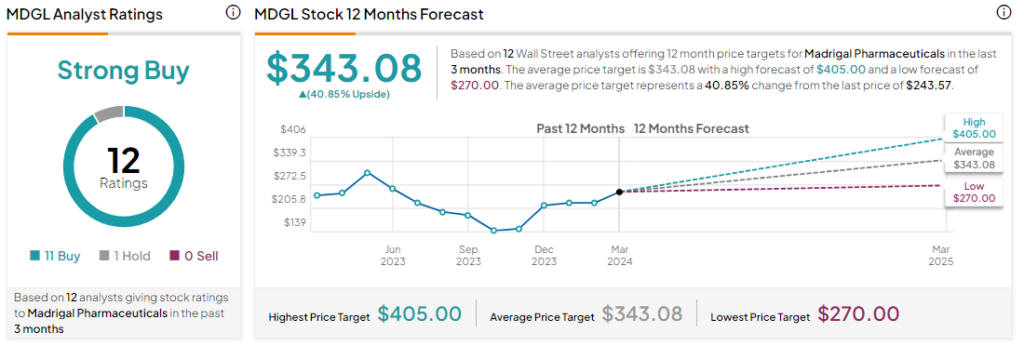
What is the Price Target for Madrigal Pharmaceuticals?
Analysts remain bullish about MDGL stock with a Strong Buy consensus rating based on 11 Buys and one Hold. Year-to-date, MDGL stock has gained by more than 5% and the average MDGL price target of $343.08 implies an upside potential of 40.8% at current levels.