Shares of pharmaceutical company Karyopharm Therapeutics (NASDAQ:KPTI) are in focus today after it bagged a fast-track designation from the U.S. Food and Drug Administration (FDA) for its myelofibrosis drug, Selinexor. The designation also includes primary myelofibrosis, post-essential thrombocythemia myelofibrosis, and post-polycythemia vera myelofibrosis.
The drug is currently under development in a Phase 3 study and has demonstrated promising efficacy and safety data in a prior Phase 1 combination study with ruxolitinib in treatment-naïve myelofibrosis patients. Next, top-line results from the Phase 3 trial are anticipated in 2025. Additionally, Karyopharm is also planning to expand the development of Selinexor in other JAKI-naïve settings as well.
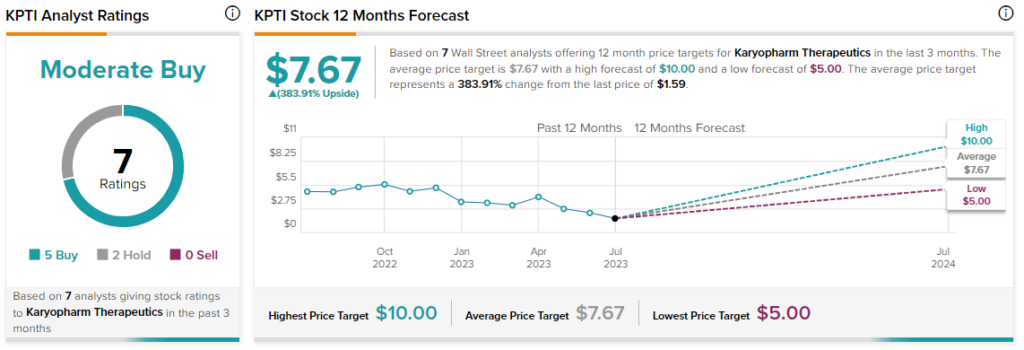
Overall, the Street has a $7.67 consensus price target on Karyopharm alongside a Moderate Buy consensus rating. This points to a massive 384% potential upside in the stock. Importantly, noting Karyopharm’s plans to expand the clinical development of selinexor in other settings, H.C. Wainwright’s Edward White has reiterated a Buy rating on the stock alongside a $10 price target today.
At the same time, short interest in the stock remains elevated at about 12.2% at present despite a 60.2% price erosion over the past 52 weeks.
Read full Disclosure
















