Iovance (NASDAQ:IOVA) stock surged about 33% in Friday’s extended trading session after its advanced melanoma treatment, Amtagvi, received accelerated approval from the U.S. Food and Drug Administration (FDA). It is worth highlighting that Amtagvi is the first tumor-infiltrating lymphocyte (TIL) cell therapy to receive FDA approval for solid tumor cancer.
Meet Your ETF AI Analyst
- Discover how TipRanks' ETF AI Analyst can help you make smarter investment decisions
- Explore ETFs TipRanks' users love and see what insights the ETF AI Analyst reveals about the ones you follow.
The regulatory approval was driven by the positive outcomes observed in Amtagvi’s Phase 2 clinical trial. The trial demonstrated a 31.5% response rate among the 73 enrolled patients. Additionally, nearly 50% of the patients showed no signs of tumor progression after 12 months of follow-up.
Remarkably, Iovance is conducting a Phase 3 trial to confirm the clinical benefits of the therapy. Iovance expects TIL therapy to start generating substantial revenue in the second quarter of this year.
Is IOVA a Good Stock to Buy?
The company is testing the potential of TIL in other solid tumor categories, such as metastatic non-small cell lung cancer. Positive developments on this front are expected to propel the company’s stock value even higher.
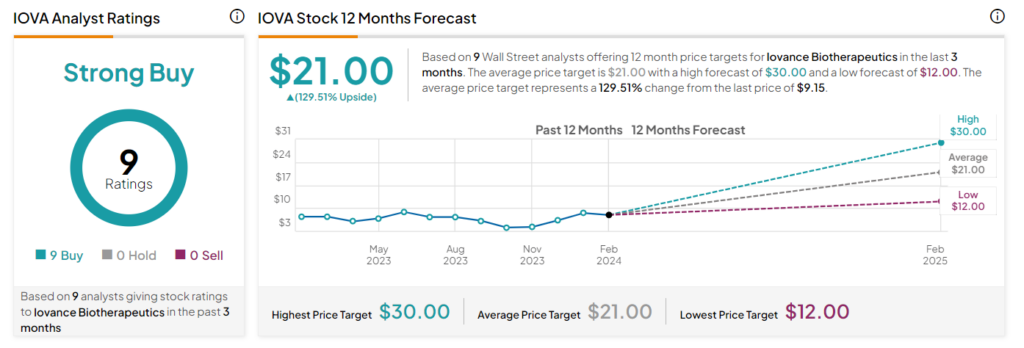
Interestingly, analysts are highly optimistic about the stock. Iovance has a Strong Buy consensus rating based on nine unanimous Buy ratings. IOVA stock has gained more than 67% over the past three months, and the average IOVA price target of $21 implies an upside potential of 129.5% at current levels.