Shares of biotechnology company Ionis Pharmaceuticals (NASDAQ:IONS) gained 7% in Thursday’s after-hours of trading. This increase follows the U.S. FDA’s (Food and Drug Administration) approval of Wainua, its nerve drug developed in collaboration with AstraZeneca (NASDAQ:AZN).
Elevate Your Investing Strategy:
- Take advantage of TipRanks Premium at 50% off! Unlock powerful investing tools, advanced data, and expert analyst insights to help you invest with confidence.
Wainua is the only approved medicine designed for treating adults with polyneuropathy caused by hereditary transthyretin amyloidosis (ATTR-PN), a rare nerve disorder. Moreover, it affects an estimated 40,000 patients globally. Further, the approval is based on Wainua’s Phase III results, demonstrating the drug’s consistent and sustained efficacy in improving neuropathy impairment.
The companies intend to commercialize Wainua to treat ATTRv-PN in the United States, with plans to pursue regulatory approval in Europe and other regions. Additionally, Wainua is expected to be available in the U.S. starting in January 2024.
With this background, let’s look at the Street’s forecast for IONS stock.
Is IONS a Good Stock to Buy?
IONS stock has delivered decent gains and is up about 30% year-to-date. Further, the company’s sustainable revenue and strong balance sheet provide a solid foundation for ongoing investments in the commercial readiness of various late-stage medicines, thereby strengthening its innovative pipeline.
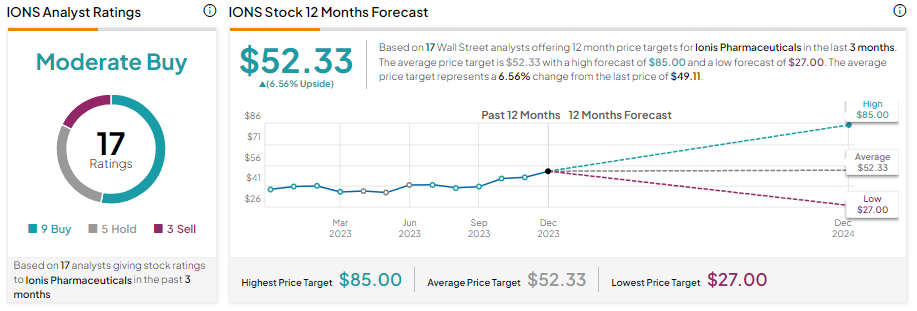
Looking ahead, Wall Street analysts are cautiously optimistic about IONS stock. With nine Buy, five Hold, and three Sell recommendations, Ionis stock has a Moderate Buy consensus rating. Moreover, analysts’ average price target of $52.33 implies an upside potential of about 6.6% from current levels.