ImmunoGen’s (Nasdaq:IMGN) Elahere drug for treating platinum-resistant ovarian cancers in adults has been granted accelerated approval by the U.S. Food and Drug Administration (FDA). Following the news, IMGN stock jumped more than 10% in extended trading hours on Monday. The stock had already risen by more than 50% in the past six months.
Meet Your ETF AI Analyst
- Discover how TipRanks' ETF AI Analyst can help you make smarter investment decisions
- Explore ETFs TipRanks' users love and see what insights the ETF AI Analyst reveals about the ones you follow.
The drug trial was conducted on 106 patients and was successful in meeting both the primary and secondary endpoints. Results show an overall response rate of 31.7% and a median duration of response of 6.9 months.
More specifically, the drug has been approved for patients with platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer, if they have received one to three prior regimens.
Full FDA approval for Elahere is dependent on the results of the Mirasol trial. Relevant data from that trial is expected in early 2023.
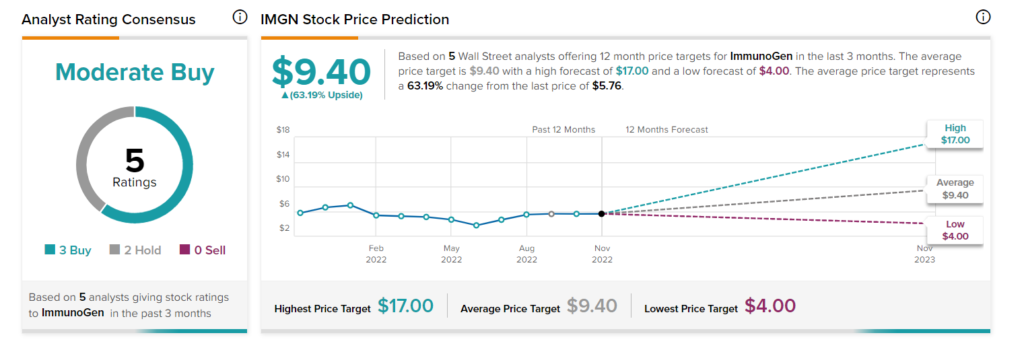
Is ImmunoGen a Good Stock to Buy?
With three Buy and two Hold ratings, ImmunoGen stock has a Moderate Buy consensus rating from analysts. On TipRanks, the average IMGN stock price forecast of $9.40 implies 63.19% upside potential to current levels.