Shares of biopharmaceutical company Hoth Therapeutics (NASDAQ:HOTH) are up in triple digits today after the U.S. Food and Drug Administration (FDA) accepted its new drug application (IND) for HT-001.
Elevate Your Investing Strategy:
- Take advantage of TipRanks Premium at 55% off! Unlock powerful investing tools, advanced data, and expert analyst insights to help you invest with confidence.
The product candidate is being developed for the treatment of rash and skin disorders arising from epidermal growth factor receptor (EGFR) inhibitor therapy. These therapeutic agents are critical in a range of cancers including pancreatic, colorectal, breast as well as non-small cell lung cancer.
Importantly, there is no approved treatment for skin toxicities related to EGFRi therapies on the market at present. Hoth now plans to initiate a Phase 2a trial of HT-001 in the first quarter of 2023.
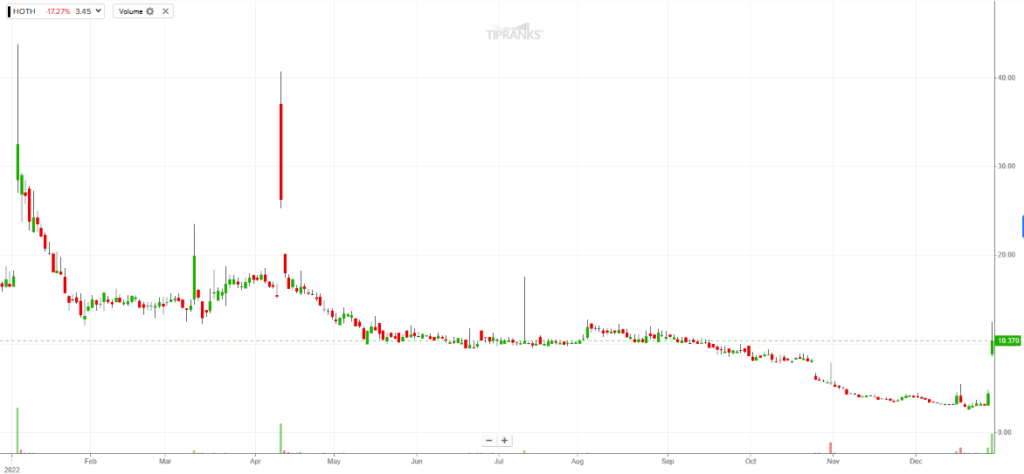
Despite today’s price gains, Hoth shares are still down nearly 73.8% over the past year. This is even after the share price gaining about 240% over the last five trading sessions.
Special end-of-year offer: Access TipRanks Premium tools for an all-time low price! Click to learn more.
Read full Disclosure
















