Shares of biopharmaceutical company First Wave BioPharma (NASDAQ:FWBI) have skyrocketed nearly 130% at the time of writing today after it reached the enrolment target in the Phase 2 SPAN trial of adrulipase for the treatment of exocrine pancreatic insufficiency in cystic fibrosis patients.
Meet Your ETF AI Analyst
- Discover how TipRanks' ETF AI Analyst can help you make smarter investment decisions
- Explore ETFs TipRanks' users love and see what insights the ETF AI Analyst reveals about the ones you follow.
The multi-center study is evaluating an enhanced enteric micro granule delivery formulation of the drug for safety, tolerability and efficacy in about 12 patients.
Further, a majority of subjects have completed dosing and the company remains on schedule for a topline data read in July. According to the National Pancreas Foundation, the number of patients with exocrine pancreatic insufficiency resulting from chronic pancreatitis currently stands at 95,000 in the U.S.
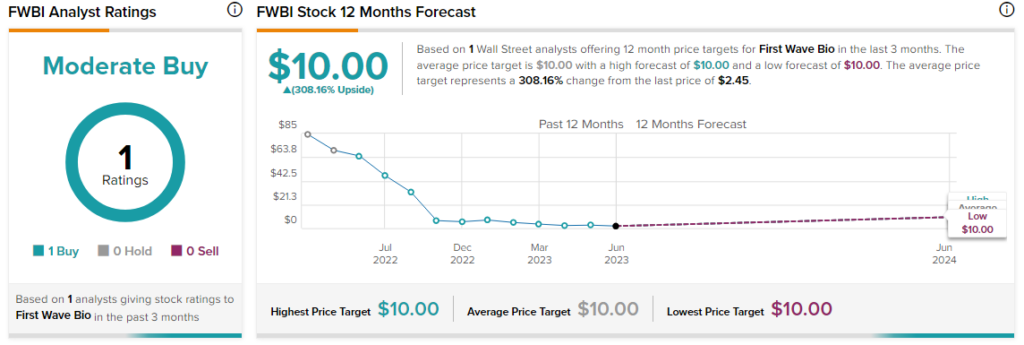
H.C. Wainwright’s Yi Chen has reiterated a Buy rating on FWBI alongside a $10 price target. This points to a massive 308% potential upside in the stock. That’s after a nearly 95% drop in FWBI shares over the past year.
Read full Disclosure
















