Shares of biopharmaceutical company First Wave BioPharma (NASDAQ:FWBI) are down 38% at the time of writing today after preliminary data from a Phase 2 trial of adrulipase in cystic fibrosis disappointed investors.
Meet Your ETF AI Analyst
- Discover how TipRanks' ETF AI Analyst can help you make smarter investment decisions
- Explore ETFs TipRanks' users love and see what insights the ETF AI Analyst reveals about the ones you follow.
Initial data from the study, which is evaluating an enhanced enteric microgranule delivery formulation of the drug in subjects with exocrine pancreatic insufficiency in cystic fibrosis showed the drug was well tolerated and demonstrated improvement as compared to its earlier formulations.
On the other hand, based on the preliminary data, it is probable that the primary efficacy endpoint of the trial was not reached. The company expects to announce additional primary and secondary endpoint findings from the data in about eight weeks.
Next, First Wave plans to engage with the U.S. Food and Drug Administration for an end-of-phase 2 meeting and evaluation of parameters for a registrational Phase 3 study of the drug.
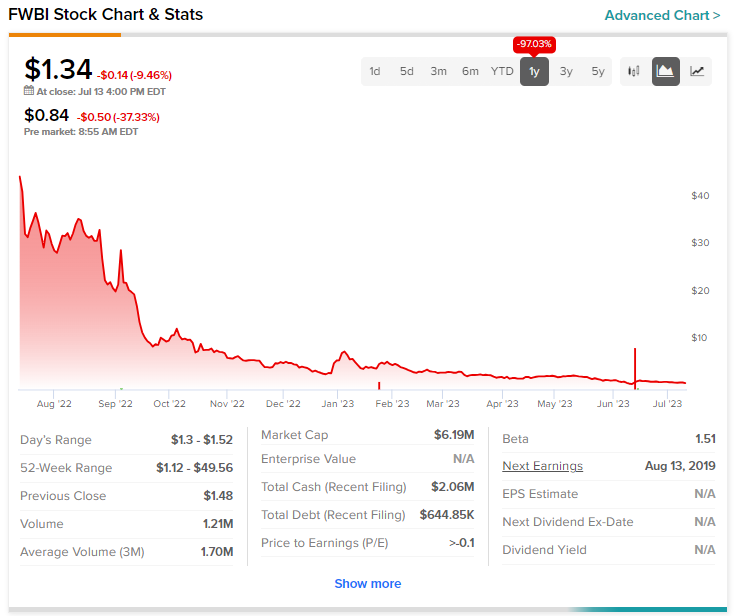
H.C. Wainwright’s Yi Chen has reiterated a Buy rating on the stock alongside a $10 price target. After a 97.1% price erosion over the past year, the price target points to a massive 646% potential upside in the stock.
Read full Disclosure
















