The U.S. Food and Drug Administration (FDA) is evaluating the effects of cannabis-derived compounds, like Cannabidiol or CBD, and is planning to make recommendations on regulating the use of CBD in food and supplements. The regulatory body intends to reveal its oversight plans in the coming months and decide whether cannabis-derived products require new FDA rules or new legislation from Congress.
Elevate Your Investing Strategy:
- Take advantage of TipRanks Premium at 50% off! Unlock powerful investing tools, advanced data, and expert analyst insights to help you invest with confidence.
Dr. Patrick Cournoye, the head of FDA’s Cannabis Product Committee, told the Wall Street Journal that the FDA wants to examine if CBD can be safely consumed for a long period or during pregnancy. Currently, the FDA says that CBD cannot be added to food or marketed as dietary supplements. Also, companies making therapeutic claims about their CBD products must prove with clinical trials that they meet the FDA’s standard for new drugs. Note that Jazz Pharmaceutical’s (JAZZ) Epidiolex is the only FDA-approved CBD medicine.
Growing Concerns About Cannabis-Derived Products
The cannabis plant contains several compounds or cannabinoids, including tetrahydrocannabinol (THC) and CBD. While THC is psychoactive and causes the high associated with the use of marijuana, CBD isn’t psychoactive. Congress legalized hemp and hemp-derived CBD products in 2018 but left their regulation to the FDA. Hemp is defined as any part of the cannabis Sativa plant with no more than 0.3% of THC.
Since hemp’s approval, makers of products like CBD oil have been functioning without specific federal rules governing the manufacturing or marketing of such products. Meanwhile, some U.S. states have established their own rules for CBD products.
Norman Birenbaum, a senior adviser with the FDA working on this matter, pointed out that a number of intoxicating hemp-derived cannabinoids have been launched over the past one and a half years. The agency is considering regulating products that pose an immediate public-health risk, like candies that could be accidentally consumed by children. A child in Virginia died in May 2022 after eating Delta-8 gummies.
Shares of some U.S.-listed cannabis companies were down on Tuesday following the WSJ report about potential regulations for cannabis-derived products. Let’s have a look at two stocks that declined in reaction to this latest development.
Is CRLBF a Good Stock to Buy?
Multi-state operator Cresco Labs (CRLBF) has operations in ten U.S. states. The company’s extensive CBD offerings include Mindy’s edibles, Good News gummies, and Wonder Wellness Co. gummies.
Wall Street is cautiously optimistic about Cresco stock, with a Moderate Buy consensus rating based on five Buys and two Holds. At $6.56, the average CRLBF stock price target implies about 275% upside potential. Shares tumbled 7.7% on Tuesday and have plunged nearly 74% year-to-date.

What is the Price Target for CURLF?
Curaleaf Holdings (CURLF) is a multi-state operator with an extensive presence in 21 states. The company offers several CBD products, including Select CBD Bites, Curaleaf Hemp lip balm and other cosmetics, and Plant Precision gummies.
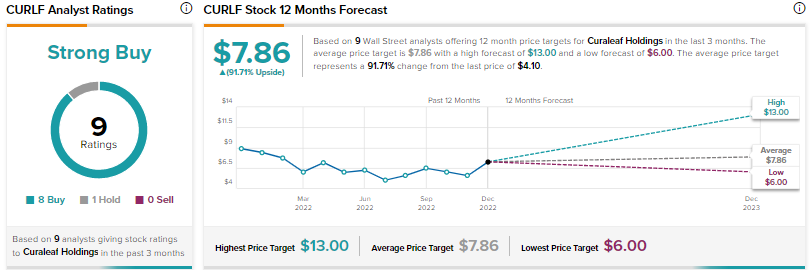
Wall Street’s Strong Buy consensus rating for Curaleaf stock is based on eight Buys and one Hold. The average CURLF price target of $7.86 implies nearly 92% upside potential. Shares fell 4.4% on Tuesday and have declined over 54% so far this year.
Special end-of-year offer: Access TipRanks Premium tools for an all-time low price! Click to learn more.
















