Shares of biopharmaceutical company Biohaven (NYSE:BHVN) are nosediving today after the U.S. Food and Drug Administration refused to review the NDA application for troriluzole in spinocerebellar ataxia (SCA) as the study failed to reach its primary endpoint.
Elevate Your Investing Strategy:
- Take advantage of TipRanks Premium at 50% off! Unlock powerful investing tools, advanced data, and expert analyst insights to help you invest with confidence.
The company is now planning to request a Type A meeting with the healthcare regulator to address its concerns. Data from a 1-year study duration had pointed to an 80% decrease in disease progression and the company believes the drug leads to significant benefits including delayed disease progression and a decrease in falls.
Separately, Biohaven is also evaluating troriluzole for the treatment of obsessive-compulsive disorder (OCD) and plans to complete enrollment in a Phase 3 trial by the end of 2023.
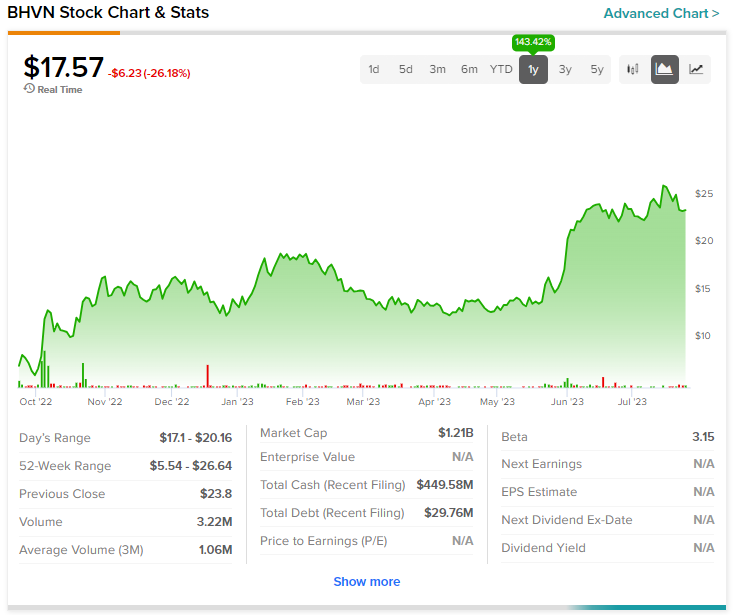
Overall, the Street has a $28 consensus price target on Biohaven alongside a Moderate Buy consensus rating. Despite today’s mega price erosion, shares of the company are still up nearly 143.4% over the past year.
Read full Disclosure