Biogen (BIIB) recently inked a license and partnership agreement with InnoCare Pharma Limited. Biogen will gain exclusive rights to Orelabrutinib for the worldwide treatment of multiple sclerosis (MS) and few other autoimmune diseases outside of China (including Hong Kong, Macau and Taiwan). InnoCare will keep its exclusive rights to use Orelabrutinib to treat cancer and other autoimmune diseases in China.
Meet Your ETF AI Analyst
- Discover how TipRanks' ETF AI Analyst can help you make smarter investment decisions
- Explore ETFs TipRanks' users love and see what insights the ETF AI Analyst reveals about the ones you follow.
Orelabrutinib is a small molecule Bruton’s tyrosine kinase inhibitor (BTKi) that is administered orally to patients of MS, cancer, and certain other autoimmune diseases as a potential treatment. Its ability to cross the blood-brain barrier makes it an excellent candidate for MS treatment. Notably, study is still underway on Orelabrutinib in a multi-country, placebo-controlled Phase 2 trial in relapsing-remitting MS.
Per the agreement, InnoCare will receive $125 million as an upfront payment. Apart from this, as the collaboration progresses, InnoCare might also get paid up to $812.5 million upon achieving potential development milestones and in potential commercial payments. Furthermore, InnoCare is eligible to receive tiered royalties of 13%-19% on expected net sales of any product as a result of the collaboration.
Biogen’s three-decade-long extensive research on MS has led to a portfolio of effective MS products. (See Biogen stock chart on TipRanks)
Alfred Sandrock, Jr., M.D., Ph.D., Head of Research and Development at Biogen, said, “Given the complex and chronic nature of MS, we believe the unique characteristics of Orelabrutinib, combining high selectivity and CNS penetrance, may translate to potential clinical advantages relative to other BTKi programs.”
Moreover, Jasmine Cui, Ph.D., Co-Founder, Chairwoman and Chief Executive Officer, InnoCare, said, “Biogen is a recognized leader in neuroscience and we believe this transaction will help advance the development of Orelabrutinib in MS.”
On July 9, FDA commissioner Janet Woodcock confirmed her request for an OIG (Office of Inspector General) investigation into the possible under-the-table interactions between FDA and Biogen regarding the review process of its controversial Alzheimer’s drug, Aducanumab (marketed as Aduhelm). Following this, Stifel Nicolaus analyst Paul Matteis reiterated a Buy rating on Biogen, with a price target of $443, implying a 26.9% upside potential to current levels.
Matteis said the “headline looks awful,” referring to Dr. Woodcock’s letter to the OIG. The analyst believes that Dr. Woodcock’s letter seems to be a move to remove pressure from the FDA. He further said that despite working up Biogen’s shareholders, Dr. Woodcock’s investigation request is not expected to materially impact Aducanumab’s marketing authorization.
Matteis also believes that as Biogen ramps the launch of Aduhelm, the fundamentals of the company will drive its shares, and shareholders will move out of theoretical fears.
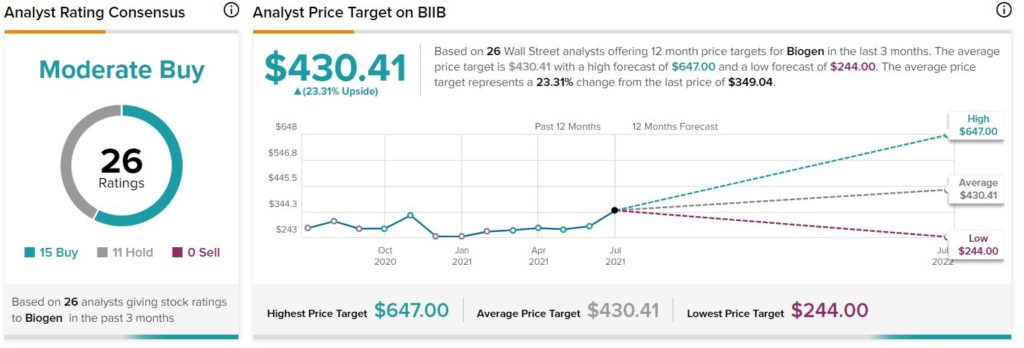
Consensus among analysts is a Moderate Buy based on 15 Buys and 11 Holds. The average Biogen price target of $430.41 implies 23.3% upside potential to current levels.
Recent News:
Houlihan Lokey Snaps up Baylor Klein
General Motors Preparing to Shock EV Industry
BMO Expands 2021 Grant Program for Women-Owned Businesses
















