The U.S. Food and Drug Administration (FDA) has granted full approval to Biogen (NASDAQ:BIIB) and Eisai’s (ESALF) Alzheimer’s treatment Leqembi, making it the first antibody treatment to receive full approval for the fatal brain disorder.
Elevate Your Investing Strategy:
- Take advantage of TipRanks Premium at 50% off! Unlock powerful investing tools, advanced data, and expert analyst insights to help you invest with confidence.
Biogen, Eisai’s Alzheimer’s Drug
In January, Leqembi received the FDA’s accelerated approval due to its ability to clear sticky deposits of a protein called amyloid beta from the brains of Alzheimer’s patients. The treatment slowed cognitive decline in patients with early Alzheimer’s disease by 27% compared with a placebo in an 18-month study.
With the full approval, patients enrolled for Medicare will now be eligible for broad coverage for the treatment with the drug, if they qualify and agree to report data to a registry so that the safety and effectiveness of the drug can be tracked. With a list price of $26,500, the drug is very expensive to afford without insurance coverage.
Eisai expects Leqembi to generate $7 billion in worldwide sales annually by 2030. The company had earlier projected that 100,000 Alzheimer’s patients in the U.S. could use anti-amyloid drugs in two to three years.
However, there are some concerns about the safety of the drug, as the treatment carries risks of brain swelling and bleeding. In fact, three patients who participated in Eisai’s study died. However, FDA scientists said that it can’t be clearly established if Leqembi played a role in these deaths.
It is important to note that Leqembi’s label will have a boxed safety warning, highlighting the risk of life-threatening brain swelling for Alzheimer’s drugs in the same class. The new label explains why patients should be monitored for potentially dangerous brain swelling and bleeding associated with amyloid-lowering antibodies. It further warns that the risk is higher in patients with two copies of a gene, APOE4, associated with Alzheimer’s and that while genetic testing is highly recommended, it is not mandatory.
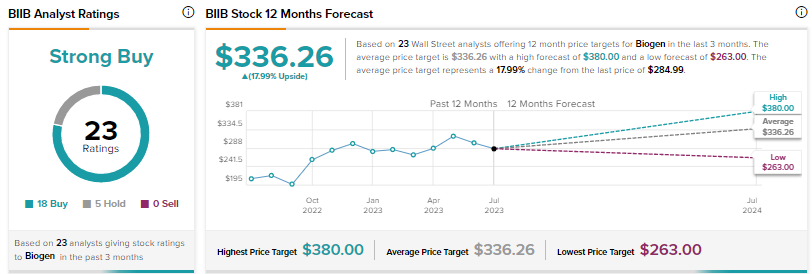
Is Biogen a Buy, Sell, or Hold?
Following the announcement, Mizuho analyst Salim Syed said that the updated label didn’t have any major surprises and the changes were generally as expected, with “ just some additional emphasis and consideration for certain risk groups (which is expected after the adcom commentary).”
The analyst added that he did find some commentary around APOE4 testing more interesting, saying “deeper in the label the FDA actually briefly comments on APOE4 tests themselves — something to think about perhaps as it relates to the commercial ramp.” Syed reiterated a Buy rating on Biogen with a price target of $340.
Biogen scores a Strong Buy consensus rating based on 18 Buys and five Holds. The average price target of $336.26 implies about 18% upside. Shares have advanced nearly 3% since the start of this year.