Penny stocks are controversial, to say the least. When it comes to these under $5 per share investment opportunities, Wall Street observers usually either love them or hate them. The penny stock-averse point out that while the bargain price tag is tempting, there could be a reason shares are trading at such low levels like poor fundamentals or insurmountable headwinds.
Meet Your ETF AI Analyst
- Discover how TipRanks' ETF AI Analyst can help you make smarter investment decisions
- Explore ETFs TipRanks' users love and see what insights the ETF AI Analyst reveals about the ones you follow.
However, the other side of the coin has merit as well. Naturally, with these cheap tickers, you get more bang for your buck in terms of the amount of shares. On top of this, other more expensive and well-known names aren’t as likely to produce the colossal gains that penny stocks are capable of.
Given the nature of these investments, Wall Street analysts recommend doing some due diligence before pulling the trigger, noting that not all penny stocks are bound for greatness.
Bearing this in mind, we used the TipRanks database to pinpoint two penny stocks that have received enough support from the analysts to earn a “Strong Buy” consensus rating. The cherry on top? Each of the tickers boasts substantial upside potential of over 200%.
aTyr Pharma (LIFE)
We’ll start with aTyr Pharma, a clinical-stage biotech company working to develop new treatments for a range of conditions, from fibrosis, to inflammation, to various cancers. The company’s research and development program uses a tRNA synthetase platform to translate theory into new therapeutic agents. aTyr has an extensive portfolio of intellectual property, giving it exclusive use of its extracellular tRNA synthetase protein fragments.
aTyr’s leading drug candidate is efzofitimod, formerly known as ATY1923. Efzofitimod is a fusion protein, built on a combination of immunomodulatory domain of histidyl-tRNA synthetase fused to the FC region of a human antibody. The drug candidate has been developed as a treatment for pulmonary sarcoidosis, a major form of interstitial lung disease, and currently has two leading clinical trials of note for investors. The more advanced trial is a pivotal Phase 3 study, on the randomized double-blind placebo-controlled model, testing the drug candidate for 52 weeks as a treatment for pulmonary sarcoidosis. The study aims to enroll up to 264 patients in the US, Europe, and Japan, and enrollment is ongoing.
Along with this, aTyr has plans to launch a Phase 2 study, as proof-of-concept in the treatment of SSc-ILD, or systemic sclerosis associated with interstitial lung disease. This study is expected to begin later this year, having received IND clearance in Q4 of last year. The study will last 28 weeks, with three parallel cohorts.
The company is partnered with Kyorin Pharmaceutical in Japan, for the Japanese portions of the studies, and in 4Q22 aTyr received a $10 million milestone payment from Kyorin, pursuant to the licensing agreement.
Given the strength of aTyr’s platform and its $1.96 share price, several members of the Street believe that now is the time to pull the trigger.
Standing squarely in the bull camp, RBC analyst Gregory Renza cites several reasons to support his optimistic stance.
“The efzofitimod programs proceed with pivotal ILD-sarcoidosis EFZO-FIT trial enrollment underway and recent IND clearance for the ph.II trial in SSc-ILD paving the way for a start this year. We had an opportunity to catch up with management whose focus on execution, in our view, sets up a dual track and potentially broader opportunity longer-term for efzo; additional MOA data and clinical insights first at ATS in May, and over the year, keep the asset’s differentiated profile and clinical positioning in sight. We continue to see LIFE as an undervalued story in the attractive pulmonary & inflammation spaces,” Renza wrote.
In line with his bullish take, Renza rates LIFE an Outperform (i.e. Buy), with a price target of $19 that indicates his confidence in an enormous 870% upside for the next 12 months. (To watch Renza’s track record, click here)
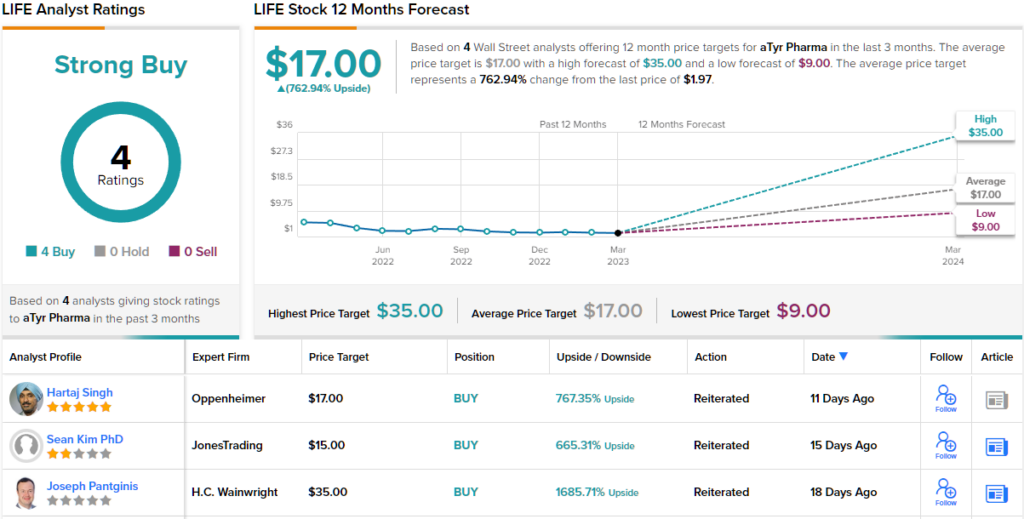
Overall, the bulls are definitely out and running for this stock, as the 4 recent analyst reviews are all positive – for a unanimous Strong Buy consensus rating. Additionally, the $17 average price target brings the upside potential to ~763%. (See LIFE stock forecast on TipRanks)
Cue Biopharma, Inc. (CUE)
Next up is Cue, a clinical-stage biopharma company working to improve the efficacy of immunotherapy cancer treatments. The company has identified a serious problem: while immunotherapies have shown promise, they only bring a benefit to some 15% to 20% of cancer patients. Cue is working on a new class of injectable biologic drugs, a set of novel therapeutic agents designed to selectively engage and modulate disease-specific T cells. These drug candidates are developed on the company’s proprietary platform, Immuno-STAT. The aim is to reach more patients and expand the benefits available from more effective, better tolerated, cancer therapeutics.
The new therapeutics will act through mimicry of the body’s natural immune system signals, or ‘cues;’ hence the company’s name and stock ticker. Cue’s stable of drug candidates, by acting in a more precisely targeted mode, will avoid some of the problems inherent in immunological cancer treatments, namely systemic and indiscriminate immune activation, which can lead to complications such as toxicity, poor efficacy, and serious adverse effects.
Cue’s development pipeline features a leading candidate, CUE-101, which is in currently undergoing multiple Phase 1 clinical trials in the treatment of HPV+ cancers, particularly head and neck squamous cell carcinoma (HNSCC). The leading trial is a monotherapy study against HPV+ recurrent/metastatic HNSCC, for which CUE-101 has already been granted Fast Track designation by the FDA.
Also covered by the FDA’s Fast Track designation is a second Phase 1 trial of CUE-101 in combination with Merck’s Keytruda. Cue has presented, in November of last year, positive data from this trial at the Society for Immunotherapy of Cancer’s annual meeting. The company aims to establish the parameters of a potential registrational trial for CUE-101 as a monotherapy by mid-2023.
Furthermore, in Q4 of last year, Cue initiated a Phase 1 trial of a second drug candidate, CUE-102, after receiving the FDA’s acceptance of the IND application. The trial will study CUE-102 as a treatment for Wilms’ Tumor 1 (WT1)-expressing cancers. The company has initiated patient dosing in the dose escalation monotherapy portion of the trial.
Among Cue’s fans is JMP analyst Reni Benjamin who writes: “With objective responses seen with CUE-101 monotherapy, a combination trial with pembrolizumab (pembro) showcasing tumor regressions higher than either agent alone, a dose-escalation study of CUE-102 underway, a versatile platform technology to address multiple targets in oncology and autoimmune disease, a new research partnership with Ono Pharmaceuticals, and a solid cash position, we believe Cue represents a unique investment opportunity whose shares are attractively priced…”
To this end, Benjamin rates Cue an Outperform (i.e. Buy), along with a $15 price target. This target implies shares could climb ~378% higher in the next twelve months. (To watch Benjamin’s track record, click here)
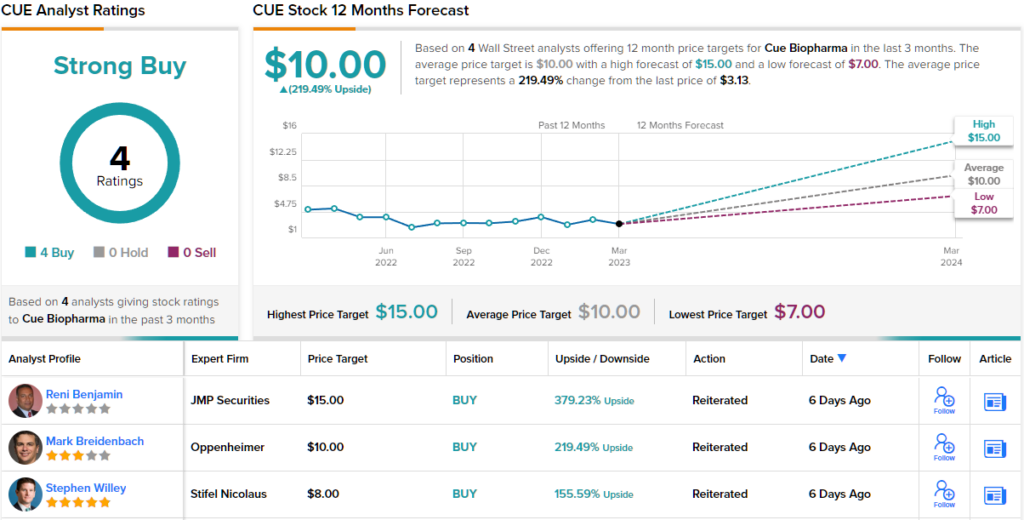
Overall, there are 4 recent analyst reviews here, all positive, giving CUE shares a Strong Buy consensus rating. The stock’s average price target of $10 implies a 219% upside potential from the current trading price of $3.13. (See Cue stock forecast on TipRanks)
To find good ideas for stocks trading at attractive valuations, visit TipRanks’ Best Stocks to Buy, a tool that unites all of TipRanks’ equity insights.
Disclaimer: The opinions expressed in this article are solely those of the featured analysts. The content is intended to be used for informational purposes only. It is very important to do your own analysis before making any investment.