Shares of biopharmaceutical company ARS Pharmaceuticals (NASDAQ:SPRY) have nosedived nearly 55% in the pre-market session today after the U.S. Food and Drug Administration (FDA) denied approval for its epinephrine nasal spray, Neffy, for the treatment of allergic reactions (Type I) in adults and children.
Pick the best stocks and maximize your portfolio:
- Discover top-rated stocks from highly ranked analysts with Analyst Top Stocks!
- Easily identify outperforming stocks and invest smarter with Top Smart Score Stocks
In the complete response letter to the new drug application for Neffy, the FDA has requested the completion of a study assessing repeat doses of Neffy in allergen-induced rhinitis conditions compared to repeat doses of an epinephrine injection product to support approval.
The response from the FDA comes against the recommendations of an advisory committee that Neffy can be approved without necessitating additional studies to support its efficacy or safety. The company expects to resubmit the application to the FDA in the first half of 2024 and anticipates an FDA action date in the second half.
Type I allergic reactions can be serious and potentially life-threatening. This necessitates immediate treatment with epinephrine. About 40 million people in the U.S. experience Type I severe allergic reactions, and the go-to treatment is an epinephrine auto-injection. If approved, Neffy could become the first needle-free nasal spray epinephrine treatment on the market.
What Is the Price Target for SPRY?
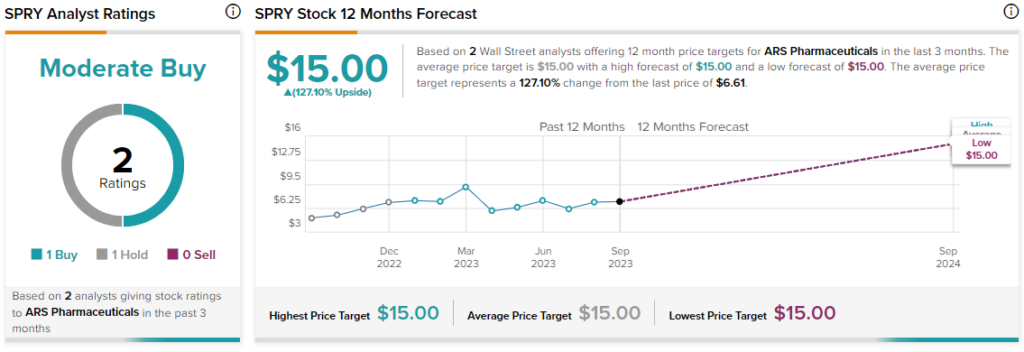
Overall, the Street has a consensus price target of $15 on SPRY, alongside a Moderate Buy consensus rating. This implies a hefty 127% potential upside in the stock.
Read full Disclosure