Shares of the global biopharmaceutical company Apellis Pharmaceuticals (NASDAQ: APLS) were up in pre-market trading at the time of writing on Monday after the company announced its GALE extension study data following 30 months of continuous treatment with SYFOVRE (pegcetacoplan injection). Syfovre is Apellis’ first and only treatment for geographic atrophy (GA) secondary to age-related macular degeneration (AMD) approved by the U.S. FDA.
Elevate Your Investing Strategy:
- Take advantage of TipRanks Premium at 50% off! Unlock powerful investing tools, advanced data, and expert analyst insights to help you invest with confidence.
Caroline Baumal, M.D., chief medical officer, Apellis commented, “The GALE results show that SYFOVRE continues to demonstrate robust and increasing effects through 30 months with both monthly and every-other-month treatment. We are especially excited by the data showing that SYFOVRE reduced GA lesion growth by up to 45% in patients with nonsubfoveal lesions.”
GALE is a Phase 3, multicenter, open-label, extension study to evaluate the long-term efficacy and safety of SYFOVRE. This study indicated that the safety profile of SYFOVRE was consistent with previously reported clinical data.
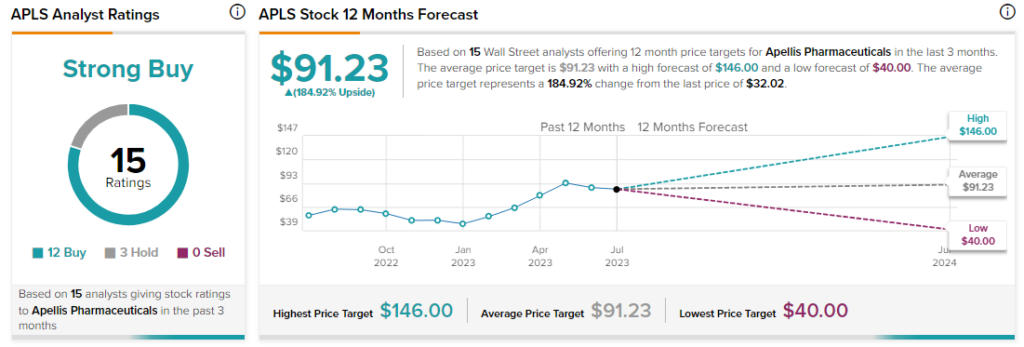
Analysts are bullish about APLS stock with a Strong Buy consensus rating based on 12 Buys and three Holds.
















