Shares of Soligenix (NASDAQ:SNGX) plunged in today’s session, which can be attributed to the FDA’s refusal to review its new drug application. The drug in question is HyBryte, which is being developed in an attempt to treat cutaneous T-cell lymphoma. The FDA believes that the application was sufficiently complete and has outlined the next steps that the company can take.
Pick the best stocks and maximize your portfolio:
- Discover top-rated stocks from highly ranked analysts with Analyst Top Stocks!
- Easily identify outperforming stocks and invest smarter with Top Smart Score Stocks
Nevertheless, CEO Christopher Schaber remains optimistic and stated that the company will work with the FDA to address its concerns in order to successfully get HyBryte reviewed.
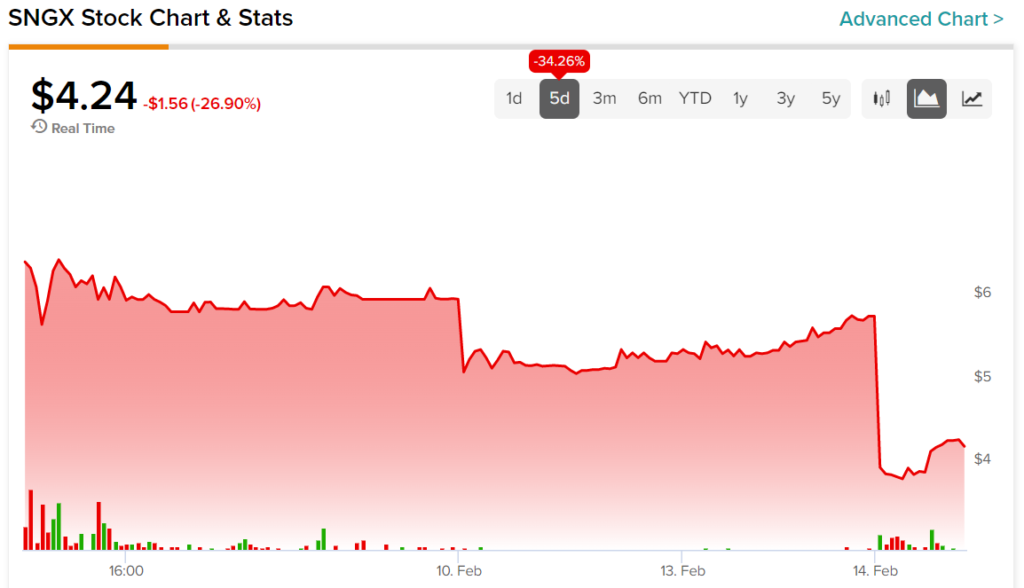
The past five trading days for SNGX investors have been frustrating. The stock saw a substantial decline on February 10. The price appeared to be recovering until today’s news sent shares even lower.



















