Drug companies Regeneron Pharmaceuticals, Inc (REGN) and Sanofi announced that the U.S. Food and Drug Administration approved its Dupixent drug for the treatment of dermatitis in children.
The Dupixent approval is for the treatment of children aged 6 to 11 years with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies.
Atopic dermatitis, the most common form of eczema, is a chronic inflammatory disease that often appears as a rash on the skin. Moderate-to-severe atopic dermatitis is characterized by rashes that can potentially cover much of the body and can include intense and persistent itching.
“This FDA approval is another milestone in the journey for Dupixent as an innovative biologic treatment for atopic dermatitis and other conditions driven in part by type 2 inflammation,” said John Reed, Global Head of Research and Development at Sanofi. “Caregivers of children with moderate-to-severe atopic dermatitis and their physicians now have access to a first-in-class biologic with a proven safety profile.”
Dupixent is a fully-human monoclonal antibody that inhibits the signalling of the interleukin-4 (IL-4) and interleukin-13 (IL-13) proteins, and is not an immunosuppressant. Data from the Dupixent clinical trials have shown that IL-4 and IL-13 are key drivers of the type 2 inflammation that plays a major role in atopic dermatitis, asthma and chronic rhinosinusitis with nasal polyposis (CRSwNP). More than 150,000 patients globally have been treated with Dupixent, the company said.
George D. Yancopoulos, President and Chief Scientific Officer at Regeneron said that the two companies continue to test Dupixentin even younger children with uncontrolled moderate-to-severe atopic dermatitis from 6 months to 5 years old, as well as in children with uncontrolled, persistent asthma.
“Additionally, we are investigating Dupixent in other diseases driven by type 2 inflammation including eosinophilic esophagitis, food and environmental allergies, chronic obstructive pulmonary disease and other dermatologic diseases,” Yancopoulos said.
Shares in Regeneron have jumped 46% so far this year. The stock declined this week after the U.S. biotech company announced that it will repurchase $5 billion of common stock held by its partner Sanofi.
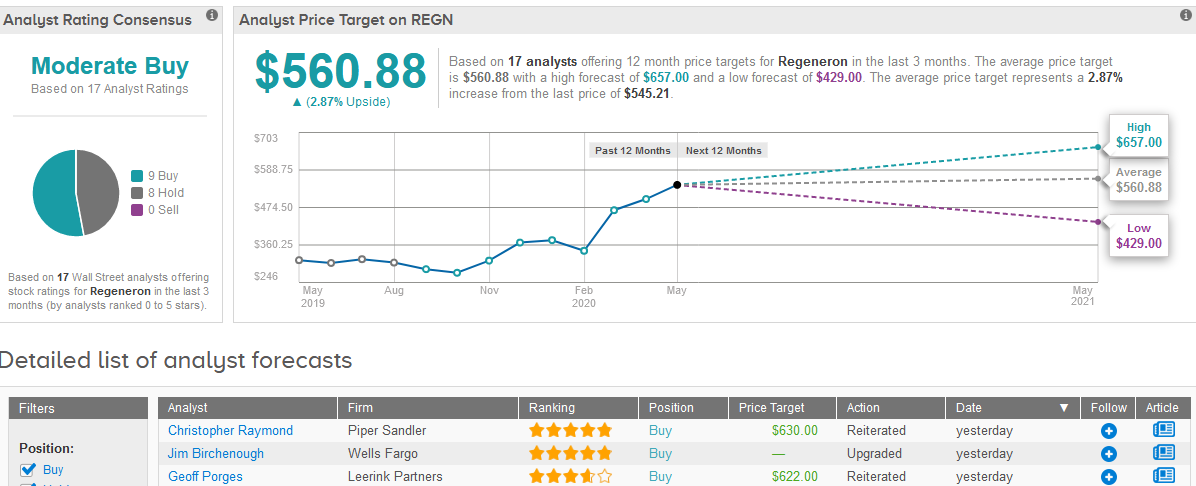
Following the news, four-star analyst Geoff Porges at Leerink Partners maintained his Buy rating on the stock with a $622 price target.
“In our view, that these shares are, or were, owned by Sanofi, or another institution, makes little difference to the value per share of Regeneron’s stock,” Porges wrote in a note to investors.
What does the rest of the Street have to say? The 17 analysts are divided between 9 Buy ratings and 8 Hold ratings adding up to a Moderate Buy consensus. In view of the stock’s recent rally, the analysts’ $560.88 average price target is less optimistic than Porges indicating a mere 2.9% upside potential in the coming 12 months. (See Regeneron stock analysis on TipRanks).
Related News:
Regeneron Announces Secondary Offering Pricing At $515/Share
Regeneron To Repurchase $5 Billion Stake From Sanofi
Gilead’s Remdesivir Most ‘Beneficial’ In Covid-19 Patients Who Need Extra Oxygen, Study Shows