Shares of Iovance Biotherapeutics (NASDAQ: IOVA) jumped in pre-market trading on Monday after the late-stage biotechnology company announced it has completed its rolling Biologics License Application (BLA) submission to the U.S. Food and Drug Administration (FDA) for lifileucel.
Pick the best stocks and maximize your portfolio:
- Discover top-rated stocks from highly ranked analysts with Analyst Top Stocks!
- Easily identify outperforming stocks and invest smarter with Top Smart Score Stocks
Lifileucel is a tumor-infiltrating lymphocyte (TIL) therapy intended as a treatment for patients with advanced (unresectable or metastatic) melanoma – a type of skin cancer.
Frederick Vogt, Ph.D., J.D., Interim President, and CEO stated, “Completing our BLA submission for lifileucel is a critical step forward in our journey to deliver the first individualized, one-time cell therapy for a solid tumor. Our preparations for commercialization remain on track to support a launch later this year. “
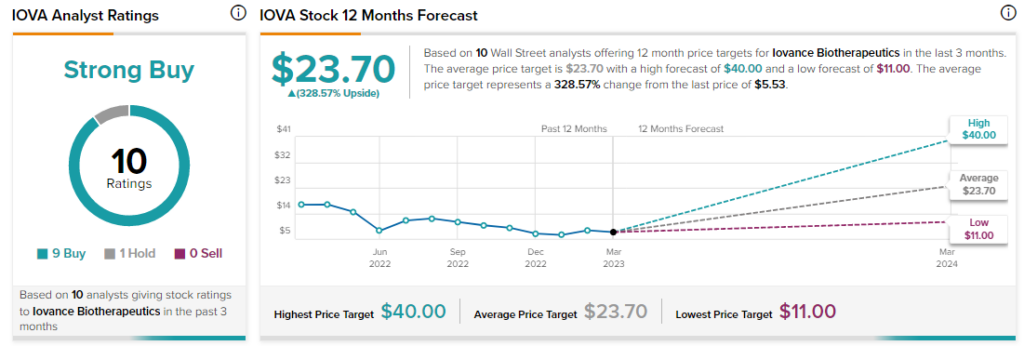
Analysts are bullish about IOVA stock with a Strong Buy consensus rating based on nine Buys and one Hold.



















