Clinical-stage biotechnology company Iovance Biotherapeutics’ (NASDAQ:IOVA) stock is sliding today after its ongoing rolling biologics License Application (BLA) for lifileucel got extended.
The U.S. Food and Drug Administration has provided feedback associated with the supplemental assay validation information and comparability data for the drug which is being targeted for the treatment of advanced melanoma.
The company is working towards addressing the comments and now expects the BLA to be completed in Q1 2023.
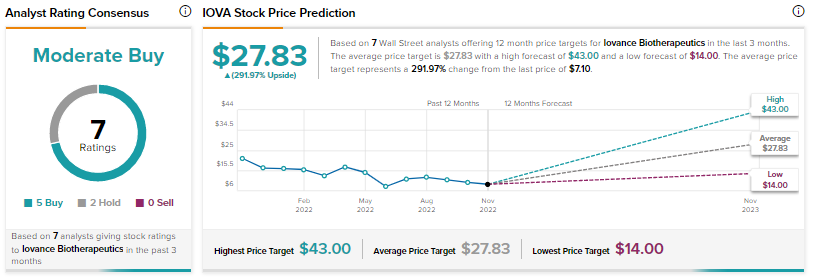
Further, a Phase 3 trial of the drug in combination with pembrolizumab in frontline advanced melanoma is anticipated to commence in late 2022.
Read full Disclosure









