Koninklijke Philips (PHG) said it has received emergency use authorization (EUA) from the U.S. Food and Drug Administration (FDA) for its monitors and displays to be used for Covid-19 patients in U.S. hospitals.
The technology company said the emergency use authorization is for its IntelliVue Patient Monitors MX750 and MX850 as well as its IntelliVue Active Displays AD75 and AD85. The patient monitoring products help to collate infection-control protocols and remotely provide patient information for caregivers, which are much needed when caring for hospitalized COVID-19 patients, the company said.
The FDA authorization enables the company to start delivering the new remote patient monitoring products to U.S. hospitals. Patient monitors, which enable clinicians to remotely monitor a patient’s condition can help hospitals minimize staff exposure to the virus that causes COVID-19.
“As the world continues to battle against COVID-19, we’re committed to ramping up production of all critical solutions that can help in this time of crisis,” said Peter Ziese, General Manager, Monitoring and Analytics at Philips. “This FDA EUA for our MX750 and MX850 monitors and IntelliVue AD75 and AD85 Active Displays allows us to do that for these remote patient monitoring solutions, which are of vital need in the intensive care unit.”
Furthermore, Philips said it is significantly increasing its patient monitor production to address the demand for more intensive care unit (ICU) capacity.
Shares in Philips, which slumped to a multi-year low in March have since been on a steady gaining streak, advancing some 44%. The stock was little changed at $45.72 in midday U.S. trading.
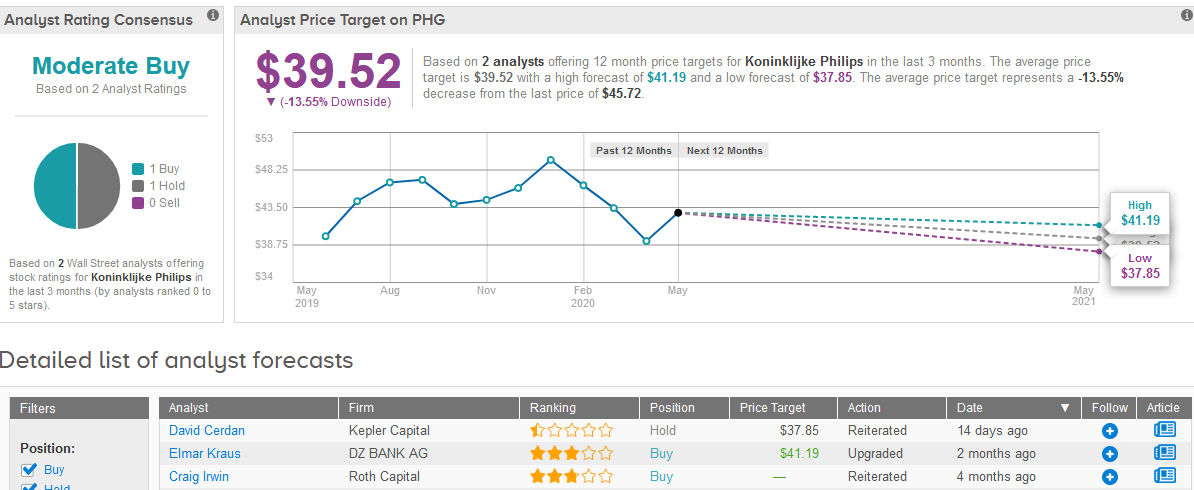
Last month, Kepler Capital analyst David Cerdan maintained a Hold rating on the stock with a $37.85 price target. Meanwhile, DZ Bank analyst Elmar Kraus upgraded Philips to Buy from Hold with a $41.19 price target.
Following the stock’s recent rally, the $39.52 average price target implies 14% downside potential in the shares in the coming 12 months. (See Philips stock analysis on TipRanks).
Related News:
Abiomed’s Heart Pump Gets FDA Emergency Use Status For Covid-19 Patients
Novavax Seeks To Make 1 Billion Covid-19 Vaccine Doses
Eli Lilly Starts Dosing Patients In World’s First Covid-19 Antibody Trial