Moderna (MRNA) has announced the initiation of a rolling submission to Health Canada for mRNA-1273, the company’s vaccine candidate against COVID-19. Shares in Moderna rose 4% on Tuesday.
This initiation follows positive results from a preclinical viral challenge study of mRNA-1273 and the positive interim analysis of the Phase 1 study of mRNA-1273 in healthy adults and older adults (ages 56-70 and 71+).
The rolling submission has been accepted under the Canadian Minister of Health’s Interim Order, which permits companies to submit safety and efficacy data as soon as they become available. This rolling review process allows Health Canada to begin its assessment and accept new evidence as it becomes available until the application is deemed complete.
Health Canada says that it will not make a decision to authorize any vaccine being considered under rolling review until it has received all necessary evidence to support its safety and efficacy. Following the authorization of any vaccine submission, Health Canada will publish the evidence used in making its decision for transparency.
On September 22, the Canadian government increased its confirmed order commitment to 20 million doses of mRNA-1273. Moderna remains on track to be able to deliver up to 56 million doses of its COVID-19 vaccine to Canada beginning in 2021.
“We are pleased with the interactions with the Canadian regulatory authorities and we appreciate their guidance and confidence in Moderna to pursue a rolling submission in Canada for our COVID-19 vaccine candidate, mRNA-1273,” stated CEO Stéphane Bancel.
mRNA-1273 is currently being studied in a Phase 3 randomized, 1:1 placebo-controlled trial of 30,000 participants at the 100 µg dose level in the U.S. As of October 9, the Phase 3 COVE study has enrolled approximately 28,618 participants with 22,194 having received their second vaccination.
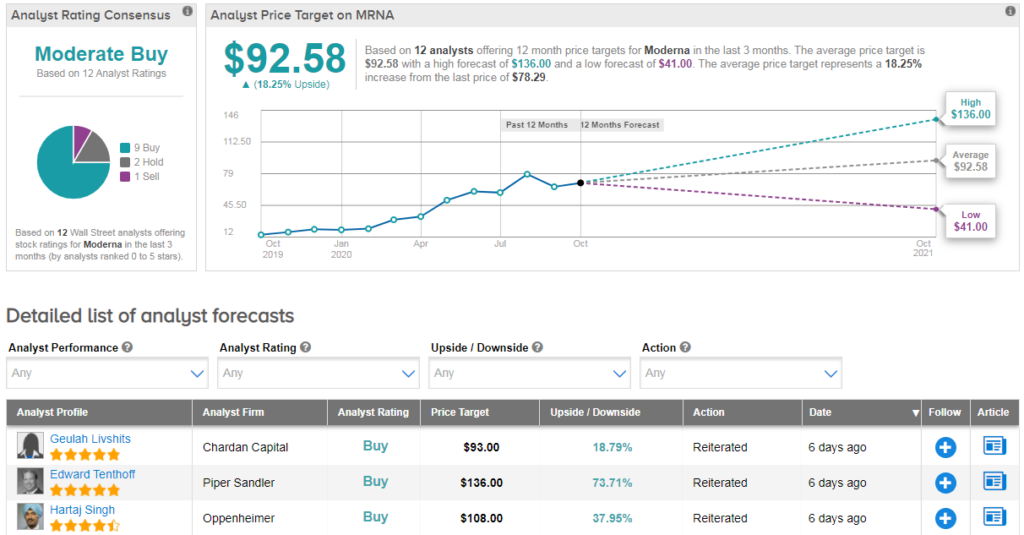
Shares in Moderna have tripled so far this year. Due to the extreme rally, Wall Street analysts have a cautiously optimistic Moderate Buy consensus on the stock’s outlook. Looking ahead, the $92 average price target suggests additional 18% upside potential lies ahead.
Speaking for the bulls, Oppenheimer analyst Hartaj Singh recently reiterated his buy rating on the stock with a $108 price target.
“We continue to like MRNA as we enter the 3Q20 reporting cycle, as we expect SMID and large-cap biotech performance to bifurcate. Over the remainder of 2020, MRNA could begin to provide preliminary color on how sales/earnings could evolve for investors for 2021 as well as oneoff nature of the pandemic’s initial revenue stream” he explained. (See MRNA stock analysis on TipRanks).
Related News:
PerkinElmer Raises 3Q Sales Outlook Fueled By COVID-19 Testing Demand
J&J Halts Covid-19 Vaccine Trial Due To ‘Unexplained Illness’
Eli Lilly Pauses Late-Stage Covid-19 Antibody Trial Over Safety Concerns









