Johnson & Johnson (JNJ) initiated first-in-human safety trials of its COVID-19 vaccine candidate after a single dose in pre-clinical studies showed “robust protection”.
The pre-clinical study data disclosed that J&J’s investigational vector-based vaccine triggered a robust immune response and neutralizing antibodies, successfully preventing subsequent infection and providing complete or near-complete protection in the lungs from the virus in non-human primates (NHPs). Based on the data, J&J kicked off a Phase 1/2a first-in-human clinical trial of the vaccine candidate, Ad26.COV2.S, in healthy volunteers in the US and Belgium.
“We are excited to see these pre-clinical data because they show our SARS-CoV-2 vaccine candidate generated a strong antibody response and provided protection with a single dose,” said J&J’s Chief Scientific Officer Paul Stoffels. “The findings give us confidence as we progress our vaccine development and upscale manufacturing in parallel, having initiated a Phase 1/2a trial in July with the intention to move into a Phase 3 trial in September.”
The Janssen COVID-19 clinical trial program, including the Phase 1/2a clinical trial and the Phase 3 clinical trial program, will evaluate both one- and two-dose regimens of Ad26.COV2.S in parallel studies. In addition, plans are underway for a Phase 2a study in the Netherlands, Spain and Germany and a Phase 1 study in Japan, the company said.
J&J said that as the company progresses the clinical development of SARS-CoV-2, it continues to boost manufacturing capacity and is in active discussions with global strategic partners to support worldwide access. This is part of the company’s target to supply more than 1 billion doses globally through the course of 2021, provided the vaccine is safe and effective.
Shares have been on a steep gaining path since plunging to a multi-year low in March and are now trading less than 1% higher than at the start of year.
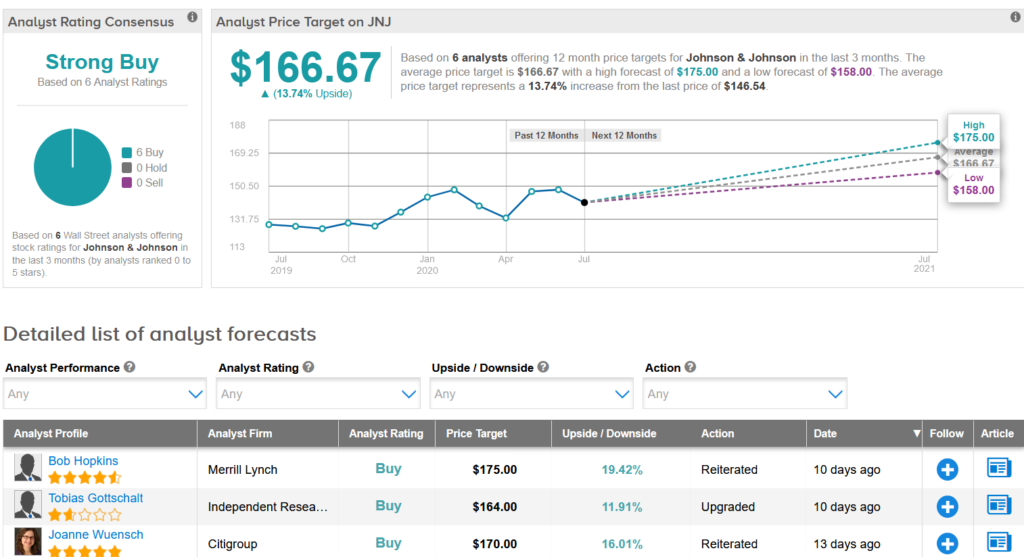
Earlier this month, Merrill Lynch analyst Bob Hopkins reiterated a Buy rating on the stock with a $175 price target, saying that despite JNJ’s strong fundamentals the stock trades at a discount to the S&P500 which he calls a “relative mispricing”.
“We continue to like the risk reward in JNJ,” Hopkins wrote in a note to investors. “While visibility remains low with litigation, Covid’s impact on the economy could well bring names like JNJ back into favor and goodwill from a successful vaccine could limit drug pricing risk.”
Overall, the rest of the Street shares Hopkins’ bullish outlook on the stock. The Strong Buy analyst consensus boast 6 unanimous Buy ratings. That’s with a $166.67 average analyst price target indicating upside potential of 14% in the coming 12 months. (See JNJ stock analysis on TipRanks).
Related News:
Quest Gets FDA Nod For Faster Covid-19 Testing; Analyst Flips To Buy Call
Pfizer, BioNTech Rise As Phase 2/3 Covid-19 Vaccine Trial Kicks Off
AstraZeneca To Pay Up To $6B For Daiichi Cancer Drug Deal









