Eli Lilly (LLY) has today announced patients have been dosed in the world’s first study of a potential antibody treatment designed to fight COVID-19.
This investigational medicine, referred to as LY-CoV555, is the first to emerge from the collaboration between Lilly and AbCellera to create antibody therapies for the prevention and treatment of COVID-19.
Lilly scientists developed the antibody in just three months after AbCellera and the National Institute of Allergy and Infectious Diseases (NIAID) identified it from a blood sample taken from one of the first U.S. patients who recovered from COVID-19. LY-CoV555 is the first potential new medicine specifically designed to attack SARS-CoV-2, the virus that causes COVID-19.
The first patients in the study were dosed at major medical centers in the U.S., including NYU Grossman School of Medicine and Cedars-Sinai in Los Angeles. Study J2W-MC-PYAA is a randomized, placebo-controlled, double-blind Phase 1 trial that aims to investigate the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of LY-CoV555 following a single dose administered to participants hospitalized for COVID-19.
“Antibody therapies such as LY-CoV555 may have potential for both prevention and treatment of COVID-19 and may be particularly important for groups hardest hit by the disease such as the elderly and those with compromised immune systems,” said Daniel Skovronsky, chief scientific officer of Lilly Research Laboratories.
“Later this month, we will review the results of this first human study and intend to initiate broader efficacy trials. At the same time… we also are starting large-scale manufacturing of this potential therapy. If LY-CoV555 becomes part of the near-term solution for COVID-19, we want to… [have] several hundred thousand doses available by the end of the year,” continued Skovronsky.
Should Phase 1 results show the antibody can be safely administered, Lilly expects to move into the next phase of testing, studying LY-CoV555 in non-hospitalized COVID-19 patients. The company also plans to study the drug in a preventative setting, focusing on vulnerable patient populations.
Lilly is researching multiple approaches to treating COVID-19, including examining existing LLY medicines and collaborating with two biotech companies to discover novel antibody treatments for COVID-19.
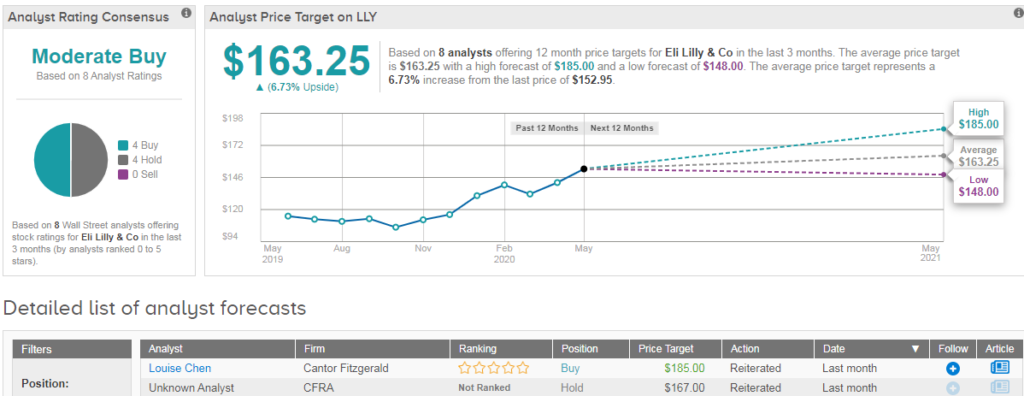
Shares in Eli Lilly are up 16% year-to-date and 2.5% in Monday’s pre-market trading. Wall Street analysts are divided evenly between 4 Buy ratings and 4 Hold ratings, which add up to a Moderate Buy consensus. The $163.25 average price target implies 7% upside potential in the coming 12 months. (See Eli Lilly’s stock analysis on TipRanks).
Related News:
BioMarin Provides Positive Gene Therapy Update For Severe Hemophilia A
Pfizer Loses 6% On Disappointing Ibrance Breast Cancer Outcome
Novavax Seeks To Make 1 Billion Covid-19 Vaccine Doses









