Eli Lilly’s (LLY) Phase 3 trial of its leading monoclonal antibody coronavirus treatment has been paused by the US FDA over potential safety concerns, the company confirmed to CNBC on Tuesday. The news was first reported by the New York Times.
“Safety is of the utmost importance to Lilly. We are aware that, out of an abundance of caution, the ACTIV-3 independent data safety monitoring board (DSMB) has recommended a pause in enrollment,” an Eli Lilly spokeswoman told CNBC.
“Lilly is supportive of the decision by the independent DSMB to cautiously ensure the safety of the patients participating in this study.”
Shares in Eli Lilly dropped 3% on the news.
Johnson & Johnson (JNJ) also halted its late-stage Covid-19 vaccine trial this week after a study participant fell ill, STAT reported.
“We must respect this participant’s privacy. We’re also learning more about this participant’s illness, and it’s important to have all the facts before we share additional information,” JNJ told STAT in a statement.
A document sent to researchers running the 60,000-patient clinical trial, and obtained by STAT, revealed that a “pausing rule” had been met, and as a result the online enrollment system was closed and an independent safety committee would be convened.
Earlier this month, Lilly announced that it had applied for emergency use authorization (EUA) for one of its Covid-19 antibody candidates, LY-CoV555, as a monotherapy for higher risk patients with mild-to-moderate symptoms. The company also presented new interim trial data for the combination therapy of LY-CoV555 and another antibody, LY-CoV016.
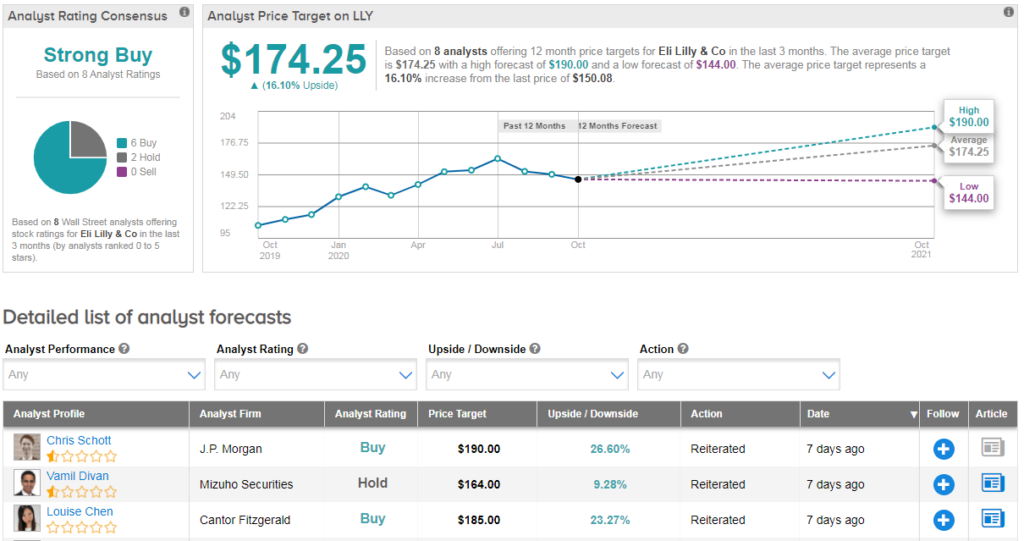
While Mizuho analyst Vamil Divan viewed the new data as “promising,” the analyst believes the “clinical meaningfulness of some datapoints remains up for debate.” Divan reiterated a Hold rating on the stock with a $164 price target.
“We wonder where the combination would fall in the Covid treatment algorithm, how/where patients would receive it, and how Lilly may price the therapy, but overall find today’s data release encouraging,” Divan wrote in a note to investors on Oct. 7. (See Eli Lilly’s stock analysis on TipRanks).
Shares in LLY are up more than 14% year-to-date and the stock scores a bullish Strong Buy Street consensus. That’s with a $174.25 average analyst price target, indicating 16% upside potential lies ahead.
Related News:
PerkinElmer Raises 3Q Sales Outlook Fueled By COVID-19 Testing Demand
J&J Halts Covid-19 Vaccine Trial Due To ‘Unexplained Illness’
Pfizer, BioNTech Initiate Rolling Canada Submission For Covid-19 Vaccine









